Professional Documents
Culture Documents
Cyclohexane Conformational Analysis
Uploaded by
휘승Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Cyclohexane Conformational Analysis
Uploaded by
휘승Copyright:
Available Formats
Cyclohexane Conformational Analysis
Cyclohexane Conformations and Energies
TABLE OF CONTENTS FOR THIS CHAPTER
CYCLOHEXANE CONFORMATIONS
CYCLOHEXANE CONFORMATIONAL ENERGY DIAGRAM
AXIAL AND EQUATORIAL BONDS
CHAIR/CHAIR INTERCONVERSION
METHYLCYCLOHEXANE CONFORMATIONS
DISUBSTITUTED CYCLOHEXANES
CYCLOHEXANE CONFORMATIONS
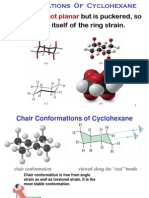
The simplest imaginable conformation for cyclohexane, the planar one, is not only not
the ground state conformation, it is so high in energy that it plays no part in the
conformational analysis of cyclohexane.This is mainly because of the large amount of
torsional strain which is present in this form. Thus, all six C-C bonds in the planar form
are eclipsed, so that we could crudely estimate the torsional strain as being at least 6
times that in eclipsed ethane, which has one eclipsed C-C bond, or 18 kcal/mole.
The ground state conformation of cyclohexane is a fully staggered conformation which
is shaped somewhat like a "chair". In this conformation there is no torsional strain at
all, and as we shall see later, no strain of any kind. Cyclohexane is unique in being the
only cyclic hydrocarbon which is completely strain-free. So the six membered ring is
the most stable of all.So we see that torsional effects, although individually small (per
C-C bond), exert a tremendously important effect on the shapes of molecules, and
shapes often effect reactivity.
In the chair conformation, four carbon atoms are coplanar, and the other two are
puckered out of this plane, one being puckered up above this plane(the carbon at the
extreme right of the depicted chair form) and one being puckered down out of this
plane(the one at the extreme left of the illustration). Another conformation which is
important in any conformational analysis is the transition state, or maximum energy
conformation on the rotational path.For cyclohexane this is the so-called "half-chair
conformation", in which now 5 carbons are co-planar, and only one is puckered out of
the plane. In the illustration above, starting from the chair conformation, the half-chair
form depicted arises from moving the left-hand-most carbon of the chair form , which
is puckered down, upward into the plane of the four coplanar atoms. This creates
considerable torsional strain, especially around the two carbon-carbon bonds involving
the left-most carbon. The energy of this conformation is about 10 kcal/mol. The
importance of this is that in any conformational changes in cyclohexane, 10 kcal is the
energy barrier, and the pathway for such conformational changes must proceed through
this relatively high energy conformation.Because this barrier is so much larger than that
in ethane or butane, cyclohexane conformational changes can be "frozen out" at, e.g.,
-78 degrees. However, they are rapid at or near room temperature. For all practical
purposes, only the chair form is populated.
There is, as in the case of butane, another energy minimum structure which can be
populated to a certain extent. This is the so- called "twist boat" conformation. However,
since this conformation is 5.5 kcal/mol higher in energy than the chair conformation, it
is not a highly populated state.Again, most of the strain energy results from bonds
which are eclipsed or partially eclipsed, i.e., from torsional strain.
CYCLOHEXANE CONFORMATIONAL ENERGY DIAGRAM
Axial and Equatorial Bonds in Cyclohexane
Note that in chair cyclohexane, there are two different types of C-H bonds, and thus
two chemically different types of hydrogens. The C-H bonds which point vertically
upward or downward are called axial. There are 6 of these, 3 upward and 3 downward
bonds, and they alternate up/down/up, etc., around the ring. The other six bonds, which
radiate away from the "equator" of the ring are called equatorial, and again there are
six, 3 of which are "slant up" and 3 of whicdh are "slant down", again alternating
around the ring.
You should be able to quickly draw cyclohexane rings in which the axial and equatorial
bonds are readily identifiable and distinguishable..
CHAIR/CHAIR INTERCONVERSION OR RING FLIP
Up to now we have not described in detail the rotational path of cyclohexane and
what is the end result. We understand that the best path (the lowest energy path)
available proceeds via the half chair and requires an energy input of 10 kcal/mol.
This transition state proceeds to a twist boat energy minimum, but this is not highly
popupulated and generally plays little or no role in cyclohexane's structure or
chemistry. However, the twist boat can interconvert with another equivalent twist
boat (via the true boat conformation as a transition state) to give another chair
structure, in which the sense of the ring puckering is reversed. This is significant in
cyclohexane itself, because in this process the axial and equatorial hydrogens are
interconverted.Since this interconversion or ring flip occrus rapidly at room
temperature, all hydrogens spend 50% of their time as axial hydrogens and 50% of
their time as equatorial hydrogens, so that on the time average all C-H bonds of
cyclohexane are equivalent. However, at any given instant, there are always two
types of hydrogen.
METHYLCYCLOHEXANE CONFORMATIONS
Since axial and equatorial bonds are non-equivalent, there are two non-equivalent
positions in which to place any substituent. We use the simple methyl group as an
example, but the same concept applies to any substituent.
Equatorial methyl cyclohexane is the more stable conformation. When the ring flip
occurs, however, it converts to axial methylcyclohexane. These two conformations
are in rapid equilibrium at room temperature, but can be frozen out as distinct
compounds at -78degrees.
The equatorial conofrmation is favored in the equilibrium by a modest amount
because the axial isomer has about 1.8 kcal/mol of steric strain. This strain arises
from the interaction of one of the hydrogens of the axial methyl group with each of
the two other axial hydrogens on the same side of the ring, as illustrated above.
Each of these steric interacgions is approximately equivalent to one gauche butane
interaction of 0.9 kcal/mole, so the total is 1.8 kcal/mol. The point of the gauche
butane comparison is that the H/H distance of the sterically hindered hydrogens is
almost exactly the same in gauche butane as with axial methyl cyclohexane, except
that there are two such H/H interactions in the latter case.
The steric interactions in axial methylcyclohexane are referred to as "1,3-diaxial
interactions", because the interactions involve two axial atoms or groups (one H and
one CH3 and the carbons bearing these atoms or groups are 1,3 related.
THE "EQUATORIALITY PRINCIPLE" PROVIDES THAT ANY
SUBSTITUENT PREFERS TO OCCUPY THE LESS STERICALLY
HINDERED EQUATORIAL POSITION, IF AT ALL POSSIBLE.IN THE
CASE OF DI- OR POLY-SUBSTITUTED CYCLOHEXANES NOT ALL
SUBSTITUENTS CAN OCCUPY ALL EQUATORIAL POSITIONS IN
EVERY ISOMER, BUT THAT ISOMER WILL BE THE MOST STABLE IN
WHICH ALL OF THE SUBSTITUENTS OCCUPY EQUATORIAL
POSITIONS(see examples below).
Disubstituted Cyclohexanes
1,4-Dimethylcyclohexane
Note that with the 1,4-disubstitution pattern, the diequatorial (mosts stable)
arrangement is what we call the "trans" isomer. in it, one substituent is "slant up"
and one "slant down". Ring flipping gives a conformation in which the methyl
substituents are anti (dihedral angle of 180) to each other, but this is still trans, and
thisconformation is less stable because it has two axial substituents.
There is another isomer of 1,4-dimethylcyclohexane, the cis isomer. Recall that cis
and trans isomers are diastereoisomers, they are not different conformations of the
same isomer and cannot be readily interconverted by a simple rotational process (a
bond would have to be broken).In the cis isomer, here, one methyl group is
equatorial and one axial, and ring flipping simply gives another equivalent
conformation. Since one methyl is axial, this costs 1.8 kcal of steric strain.
Consequently, the cis isomer is less thermodynamically stable than the trans, which
has no steric strain in the more stable conformation. The energy difference is again,
1.8 kcal/mol.
In the cis isomer, one substituent is vertically up (or down) and the other slant up (or
down). So they both point, in a general sense, in the same direction, i.e., both up or
both down.
1,3-Dimethylcyclohexane
In the 1,3-disubstitution pattern (whether it is dimethyl or any other 1,3-
dibsubstitution), both groups can only be equatorial when they are both cis. So the
cis isomer is the more stable isomer in this case. Ring flipping gives a conformation
of the cis isomer which has both methyls axial. Worse than that, they are both on the
same side of the ring, so it is not an axial methyl/axial hydrogen interaction any
longer, it is an axial methy/axial methyl interaction, which is sterically much worse.
Consequently this ring flip is too energetically difficult, and this conformer can be
neglected in he conformational analysis.
The trans-1,3-dimethylcyclohexane isomer , on the other hand, has one methyl axial
in both ring-flip conformers, so that it is less stable than the cis isomer by 1.8
kcal/mol.
Both trans-1,4-dimethylcyclohexane and cis-1,3-dimethylcyclohexane have
essentially the same energy, since neither one of them has any strain at all.
The 1,2-disubstitution pattern is very much like the 1,4 pattern, in that the two
groups can only be equatorial if they are trans, so the trans isomer is more stable
than the cis. The diaxial trans conformer has 3.6 kcal of steric strain, of course and
is much less favored than the diequatorial conformer.
The cis isomer is less stable than the trans because in it, one methyl must be axial.
The cis isomer is therefore less stable than the trans by 1.8 kcal.
The key difference between the 1,2 and 1,4 patterns, is that in the diequatorial 1,2
conformation, the methyl groups are gauche as in gauche butane (remember that
gauche essentially implies a 60 degree dihedral angle ). Consequently, this
diequatorial conformation no longer is strain free, as was the 1,4-trans diequatorial
conformation, where the methyls are very far apart. The 1,2-diequatorial isomer is
0.9 kcal/mol less stable than the 1,4-diequatorial isomer, because of this gauche
butane-like interaction (recall that the gauche isomer in butane is destabilized by
exactly this amount).
BACK TO THE TOP OF THE PAGE
ON TO THE NEXT PAGE
BACK TO THE BAULD HOME PAGE
You might also like
- Holy Week Labyrinth GuideDocument4 pagesHoly Week Labyrinth GuideEileen Campbell-Reed100% (1)
- Cyclohexane Conformational AnalysisDocument17 pagesCyclohexane Conformational AnalysiscclatrumNo ratings yet
- (Culture and History of The Ancient Near East 65) Leslie Anne Warden - Pottery and Economy in Old Kingdom Egypt-Brill Academic Publishers (2014)Document343 pages(Culture and History of The Ancient Near East 65) Leslie Anne Warden - Pottery and Economy in Old Kingdom Egypt-Brill Academic Publishers (2014)HugoBotello100% (1)
- The Historical Foundations of Law. Harold BermanDocument13 pagesThe Historical Foundations of Law. Harold BermanespinasdorsalesNo ratings yet
- Enscape Tutorial GuideDocument27 pagesEnscape Tutorial GuideDoroty CastroNo ratings yet
- Monocyclic DisubstitutedDocument3 pagesMonocyclic DisubstitutedPs SatchithNo ratings yet
- Angle Strain Torsional Strain Ring StrainDocument6 pagesAngle Strain Torsional Strain Ring StrainchuasioklengNo ratings yet
- Conformational Analysis: Understanding How Molecule Shapes Impact PropertiesDocument10 pagesConformational Analysis: Understanding How Molecule Shapes Impact PropertiesPG Chemistry PG ChemistryNo ratings yet
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Document8 pagesChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNo ratings yet
- Conformational Analysis of CyclopentaneDocument11 pagesConformational Analysis of CyclopentaneNimra MalikNo ratings yet
- Conformational IsomersDocument8 pagesConformational Isomersshirodkar_16593No ratings yet
- Cycloalkane Ring Strain and ConformationsDocument18 pagesCycloalkane Ring Strain and ConformationsbgbscgvNo ratings yet
- Chapter 3: Structure and Stereochemistry of AlkanesDocument42 pagesChapter 3: Structure and Stereochemistry of AlkanesKera Maria AllengerNo ratings yet
- Org Chem Text - Chapter 1 - Sec1-14 - 1-14Document3 pagesOrg Chem Text - Chapter 1 - Sec1-14 - 1-14TET2005No ratings yet
- Ch221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedDocument11 pagesCh221 Class 8 Chapter 4: Stereochemistry of Alkanes and Cycloalkanes, ContinuedSanjay Mani TripathiNo ratings yet
- GeneralChem LS 25 PDFDocument25 pagesGeneralChem LS 25 PDFSunil NahataNo ratings yet
- Org Lec 2Document27 pagesOrg Lec 2rupayandaripaNo ratings yet
- Chair & BoatDocument9 pagesChair & BoatpardeepbthNo ratings yet
- Chapter 4 With Video LinksDocument37 pagesChapter 4 With Video LinksDoom RefugeNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- Organic - Class 8Document41 pagesOrganic - Class 8Sajan Singh LUCKYNo ratings yet
- Module 5: Conformational analysis of alkanes and cyclohexanesDocument8 pagesModule 5: Conformational analysis of alkanes and cyclohexanesARMAN AKRAM BIN OMAR / UPMNo ratings yet
- Conformational Analysis:: Chapter - 6 Stereochemistry NotesDocument12 pagesConformational Analysis:: Chapter - 6 Stereochemistry NotesMohit KambojNo ratings yet
- Conformation & Conformational IsomersDocument4 pagesConformation & Conformational Isomerspulkit asatiNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)
- Tutorial07b OrganicCycloalkanesDocument24 pagesTutorial07b OrganicCycloalkanesHana NisrinaNo ratings yet
- BSES Topic 06 Cycloalkanes and Aromatic CompoundsDocument42 pagesBSES Topic 06 Cycloalkanes and Aromatic CompoundsJhunessa Mae JalagatNo ratings yet
- The Influence of Conformational Isomerism On Drug ActionDocument6 pagesThe Influence of Conformational Isomerism On Drug Actionhectorlope45No ratings yet
- 3.9 - Conformations of Disubstituted CyclohexanesDocument5 pages3.9 - Conformations of Disubstituted CyclohexanesHajra NaeemNo ratings yet
- Cyclo AlkanesDocument38 pagesCyclo Alkanesdinesh111180No ratings yet
- Loudon5ech07sec04 Cyclohexane ConformationsDocument8 pagesLoudon5ech07sec04 Cyclohexane ConformationsF1234TNo ratings yet
- Types of Isomerisms Chain IsomerismDocument5 pagesTypes of Isomerisms Chain IsomerismRahul DubeyNo ratings yet
- Cycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheoryDocument5 pagesCycloalkane - Bayer Strain Theory and Limitation of Bayer Strain TheorySayed AltamashNo ratings yet
- CHEM F111: General Chemistry Lecture on Conformational Isomers and CycloalkanesDocument17 pagesCHEM F111: General Chemistry Lecture on Conformational Isomers and CycloalkanesRachit ShahNo ratings yet
- Conformational Isomerism 2Document8 pagesConformational Isomerism 2ytima uniNo ratings yet
- UntitledDocument46 pagesUntitled양우경No ratings yet
- Strain 02Document7 pagesStrain 02Sherlock Wesley ConanNo ratings yet
- 2m Engl Isomerism 2021 For StudDocument96 pages2m Engl Isomerism 2021 For StudGhost ShooterNo ratings yet
- Relative Stabilities of CycloakanesDocument8 pagesRelative Stabilities of CycloakanesKamal KishoreNo ratings yet
- Asymmetric HydroborationDocument22 pagesAsymmetric HydroborationsaheedvkNo ratings yet
- DecalinsDocument25 pagesDecalinstessyNo ratings yet
- Unit_5 (1)Document42 pagesUnit_5 (1)RAVI5268No ratings yet
- UntitledDocument42 pagesUntitledRAVI5268No ratings yet
- 06 Conformational Anal 3Document13 pages06 Conformational Anal 3eraborNo ratings yet
- Alkanes, Alkenes, Alkynes, and CycloalkanesDocument162 pagesAlkanes, Alkenes, Alkynes, and CycloalkanesNurtasha AtikahNo ratings yet
- CHEM 210-1 Organic Chemistry NotesDocument26 pagesCHEM 210-1 Organic Chemistry NotesRobert GardnerNo ratings yet
- Conformations IIIDocument21 pagesConformations IIIsndNo ratings yet
- Conformations and Strains of Alkanes and CycloalkanesDocument92 pagesConformations and Strains of Alkanes and CycloalkanesMutia HadiyantiNo ratings yet
- 4 CH241 CycloalkanesDocument73 pages4 CH241 CycloalkanesLuis CastilloNo ratings yet
- Conformations IIDocument16 pagesConformations IIsndNo ratings yet
- Conformational Isomerism by MuneebDocument11 pagesConformational Isomerism by MuneebMuneeb Ur RehmanNo ratings yet
- Conformational Analysis: Carey & Sundberg: Part A Chapter 3Document56 pagesConformational Analysis: Carey & Sundberg: Part A Chapter 3swastikNo ratings yet
- Organic Chemistry: M. R. Naimi-JamalDocument81 pagesOrganic Chemistry: M. R. Naimi-JamalHamidatun NisaNo ratings yet
- Cyclic Aliphatic Compounds: NomenclatureDocument19 pagesCyclic Aliphatic Compounds: NomenclatureWinnie SantiagoNo ratings yet
- Organic Lecture 2Document27 pagesOrganic Lecture 2Dhruv RaiNo ratings yet
- IsomerDocument13 pagesIsomerAssassin's j :uNo ratings yet
- Exercise 8 - Conformational Analysis - AnswersDocument4 pagesExercise 8 - Conformational Analysis - Answers210331 Bùi Phước ChíNo ratings yet
- Tutorial 15Document5 pagesTutorial 15Pace AjjaNo ratings yet
- Chair Conformation: Newman Projection of The Boat ConformationDocument11 pagesChair Conformation: Newman Projection of The Boat ConformationSunil ChoudharyNo ratings yet
- Week 3.2 Slide DitaDocument28 pagesWeek 3.2 Slide DitaAnnisah MardiyyahNo ratings yet
- CH 03Document39 pagesCH 03Anand MurugananthamNo ratings yet
- Cyclo Al KanesDocument3 pagesCyclo Al KanesIbraim ZekirNo ratings yet
- Process Planning and Cost Estimation Question BankDocument13 pagesProcess Planning and Cost Estimation Question BanksanthoshjoysNo ratings yet
- Re15209 03-95Document8 pagesRe15209 03-95Kaushik GhoshNo ratings yet
- SAQ Ans 6Document3 pagesSAQ Ans 6harshanauocNo ratings yet
- F2 IS Exam 1 (15-16)Document10 pagesF2 IS Exam 1 (15-16)羅天佑No ratings yet
- Man 400eDocument324 pagesMan 400eLopez Tonny100% (1)
- Guidelines SLCM BWDocument60 pagesGuidelines SLCM BWpnaarayanNo ratings yet
- Oral Communication Skills Assessment Republic of the PhilippinesDocument3 pagesOral Communication Skills Assessment Republic of the PhilippinesMarissa UrnosNo ratings yet
- Manjit Thapp ResearchDocument24 pagesManjit Thapp ResearchDough RodasNo ratings yet
- Christopher Westra - Laws of Attraction PDFDocument3 pagesChristopher Westra - Laws of Attraction PDFZachary LeeNo ratings yet
- 6.1.2 The Solar SystemDocument4 pages6.1.2 The Solar System205 NursyazliyanaNo ratings yet
- T2-1 MS PDFDocument27 pagesT2-1 MS PDFManav NairNo ratings yet
- 10 - (Rahman) The Relationship Between Chest Tube Size and Clinical Outcome in Pleural InfectionDocument8 pages10 - (Rahman) The Relationship Between Chest Tube Size and Clinical Outcome in Pleural InfectionfaisaldanyaniNo ratings yet
- Islamic Center Design With Islamic ArchiDocument11 pagesIslamic Center Design With Islamic ArchiMuhammad Sufiyan SharafudeenNo ratings yet
- 1161 Article+Text 5225 1 4 20220712Document9 pages1161 Article+Text 5225 1 4 20220712Warman FatraNo ratings yet
- 02.casebook - BLDG Repairs & Maint - Chapter 1 - 2011 (Water Seepage)Document13 pages02.casebook - BLDG Repairs & Maint - Chapter 1 - 2011 (Water Seepage)Hang kong TseNo ratings yet
- Bridge Manual Retaining Walls - Section 3.62 Page 3.2-2Document1 pageBridge Manual Retaining Walls - Section 3.62 Page 3.2-2lomoscribdNo ratings yet
- DissertationDocument15 pagesDissertationNicole BradyNo ratings yet
- Exam Unit 1 Out and About 1º BachilleratoDocument5 pagesExam Unit 1 Out and About 1º Bachilleratolisikratis1980No ratings yet
- Junguian PsychotherapyDocument194 pagesJunguian PsychotherapyRene Galvan Heim100% (13)
- Bharathidasan University UG/PG Exam ApplicationDocument2 pagesBharathidasan University UG/PG Exam ApplicationOppili yappanNo ratings yet
- 2019 Indonesia Salary GuideDocument32 pages2019 Indonesia Salary Guideiman100% (1)
- PTR01 21050 90inst PDFDocument40 pagesPTR01 21050 90inst PDFЯн ПавловецNo ratings yet
- "A Study Consumer Satisfaction Towards Royal Enfield BikesDocument72 pages"A Study Consumer Satisfaction Towards Royal Enfield BikesKotresh Kp100% (1)
- wizBRAINeng20 2Document4 pageswizBRAINeng20 2Deepika AgrawalNo ratings yet
- How To Critique A Photograph - Facebook PDFDocument1 pageHow To Critique A Photograph - Facebook PDFpeterNo ratings yet
- Mark Dyczkowski and Trika Journal March 2015 Vol.1.No.1.Document10 pagesMark Dyczkowski and Trika Journal March 2015 Vol.1.No.1.Mark Dyczkoswki and Trika Journal100% (2)