Professional Documents
Culture Documents
Histologic Subtypes of Oral Squamous Cell Carcinoma: Prognostic Relevance
Uploaded by
Deyfa RehadiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Histologic Subtypes of Oral Squamous Cell Carcinoma: Prognostic Relevance
Uploaded by
Deyfa RehadiCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/6346208
Histologic subtypes of oral squamous cell carcinoma: Prognostic relevance
Article in Journal (Canadian Dental Association) · June 2007
Source: PubMed
CITATIONS READS
139 14,376
4 authors, including:
Denise Tostes Oliveira Luiz Paulo Kowalski
University of São Paulo Hospital A. C. Camargo
171 PUBLICATIONS 2,048 CITATIONS 600 PUBLICATIONS 10,904 CITATIONS
SEE PROFILE SEE PROFILE
All content following this page was uploaded by Denise Tostes Oliveira on 21 May 2014.
The user has requested enhancement of the downloaded file.
Clinical Practice
Histologic Subtypes of Oral Squamous
Cell Carcinoma: Prognostic Relevance
Contact Author
Michele Conceição Pereira, DDS, MSc; Denise Tostes Oliveira, DDS, MSc, PhD; Dr. Oliveira
Gilles Landman, MD, PhD; Luiz Paulo Kowalski, MD, PhD Email: d.tostes@fob.usp.br
ABSTRACT
Squamous cell carcinoma (SCC) is the most prevalent malignant neoplasm of the oral
cavity. The conventional tumour and several histologic subtypes of SCC present morpho-
logic features and specific behaviour when they occur in the oral mucosa. For example,
basaloid SCC is an aggressive tumour and verrucous carcinoma has the lowest invasive
and metastatic potential; however, the clinical and biologic course of these oral SCC
variants has not been completely established. Furthermore, although numerous clin-
ical and histologic features associated with oral SCC have been identified, none shows
unequivocal prognostic significance. The purpose of this article is to review the various
subtypes of oral SCC, emphasizing problems in their histologic diagnosis and prognosis.
For citation purposes, the electronic version is the definitive version of this article: www.cda-adc.ca/jcda/vol-73/issue-4/339.html
C
ancer is an important public health inherent to the patient and others associated
problem in many parts of the world, with the genetic profile of the malignant epi-
and oral cancer is among the 10 most thelial cells, which reflect tumour aggressive-
common cancers worldwide.1 According to the ness. However, none of the molecular markers
International Agency for Research on Cancer associated with the genetic alterations de-
of the World Health Organization (IARC– scribed for oral SCC have shown unequivocal
WHO), cancer rates are expected to increase prognostic or predictive significance.11,12
at an alarming rate: from 10 million new cases
The value of histologic classification of
globally in 2000 to 15 million in 2020.2
conventional oral SCC (well, moderately or
In the oral cavity, squamous cell carcinoma
poorly differentiated) is controversial, and
(SCC) is the most prevalent malignant neo-
most authors now recognize that microscopic
plasm. Despite the ready accessibility of the
oral cavity to direct examination, these malig- classification alone is poorly correlated with
nancies are often still not detected until a late outcome and response to treatment.4,11,13
stage and, as a result, the survival rate for oral Furthermore, the prognostic value of identi-
cancer has remained essentially unchanged fying histologic subtypes of oral SCC (Fig. 1),
over the past 3 decades. 3 In recent years, num- which show characteristic morphology and
erous prognostic factors associated with oral specific behaviour, has not been clearly estab-
SCC have been identified,4–12 some of them lished.14 The main clinicopathologic features
JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 • 339
––– Oliveira –––
Moderately differentiated
Well differentiated Poorly differentiated
Squamous cell carcinoma
Basaloid squamous cell carcinoma Other variants
Verrucous carcinoma
• Spindle cell
• Papillary
• Adenosquamous
• Acantholytic
• Cuniculatum
Figure 1: Histologic subtypes of oral squamous cell carcinoma.
of and prognosis associated with the most frequent sub- that might aid in more precise selection of patients for
types of oral SCC are summarized in Table 1. various treatment strategies remain inconclusive.17
This review includes a brief overview of the histo- A wide range of tumour features, including size and
logic subtypes of oral SCC, emphasizing the importance site, histologic malignant grade, perineural spread at
of microscopic diagnosis of these variants, which has the invasive front, lymphovascular invasion and tumour
important clinical implications for the prognosis of the thickness, have been described as major risk factors that
tumour. adversely affect the prognosis for patients with oral SCC.
Some of these factors involve a series of genetic altera-
Conventional Squamous Cell Carcinoma tions, which occur in proto-oncogenes and in tumour
Conventional squamous cell carcinoma (CSSC) is the suppressor genes and reflect the tumour’s aggressiveness
most frequent neoplasm arising from the oral epithelium as well as the risk of metastasis.15,18 Abnormalities in the
(Fig. 2) and the risk factors associated with its occurrence, p53 gene, frequently identified by genetic markers,12,15,16,19
such as tobacco and heavy alcohol consumption, are well are likely one of the early events in the development of
established.1,15,16 oral SCC.
Despite the continuing evolution of diagnostic and The presence of 2 or more positive regional nodes,
therapeutic methods used in oncology, clinical and histo- extracapsular extension of nodal disease and positive
pathologic factors with prognostic value for oral CSCC margin resection15 have also been correlated with poor
340 JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 •
––– Subtypes Oral Squamous Cell –––
Table 1 Comparison of clinicopathologic features and prognosis of conventional squamous cell carcinoma (CSCC), verrucous
carcinoma (VC) and basaloid squamous cell carcinoma (BSCC)
Histologic subtypes of oral cancer
CSCC VC BSCC
Clinical features Ulcerated appearance Cauliflower-like appearance Ulcerated appearance
Preferential sites Tongue Buccal mucosa Base of the tongue
Floor of the mouth Hard palate Floor of the mouth
Histopathologic Variable degree of Intense keratinization, Basaloid pattern in intimate
features keratinization, pleomorphism compressive pattern and association with a squamous
and mitotic activity minimal atypia component
Metastatic potential High Absent High (similar to poorly
differentiated CSCC)
Prognosis Poor Excellent Poor (similar to poorly
differentiated CSCC)
prognosis. Although the quality of treatment is of para- or in their destruction. The protective role of inflamma-
mount importance, most analyses of prognostic factors tory cells in the development of tumours has been dem-
have failed to show any relation between treatment and onstrated in a series of studies.18 Our own experience7
survival rate.16 Furthermore, due to the limited prog- has confirmed that cells such as eosinophils may play a
nostic value of conventional clinical tumour–nodal– protective role against tumour progression. In our ex-
metastasis (TNM) staging and histopathologic grading in perience, intense tumour-associated tissue eosinophilia
oral cancer, many patients are still over- or under-treated, obtained by morphometric analysis was an independent
resulting in significant personal and socioeconomic favourable prognostic factor for 125 patients submitted
impact. 5 Intriguingly, 2 well-established histologic pre- to surgical treatment for clinical stage II and III primary
dictive factors — tumour thickness and extracapsular SCC located within the oral cavity.
spread of nodal metastases — have not become part of According to Woolgar11 the identification of accurate
routine TNM classification.11 prognosticators in oral SCC has been hampered by the
According to Pindborg and others,4 oral SCCs are relatively small number of cases of the disease, especially
classified microscopically based on a method which in any one treatment centre; by the heterogeneity of clin-
takes into account a subjective assessment of the degree ical features, such as the extent of the disease at presenta-
of keratinization, cellular and nuclear pleomorphism tion; and, in particular, by the lack of standard clinical,
and mitotic activity. The grades are well differentiated management and laboratory protocols combined with
(grade 1), moderately differentiated (grade 2) and poorly inconsistent recording and reporting of data. We agree
differentiated (grade 3). Well and moderately differenti- with this author’s suggestion that more efficient research
ated tumours can be grouped together as low grade and and development would occur with less reliance on sub-
poorly differentiated and undifferentiated tumours as jective interpretation and the wider use of standardized
high grade. databases, automated techniques, quantitative data and
Generally, histologic grading of malignancy has lim- the ability to exchange information.
ited impact on survival rate due to the subjective nature
of the assessment of various features; the fact that the Verrucous Carcinoma
small biopsies from these neoplasms may show consider- The lesser aggressiveness of verrucous carcinoma (VC)
able histologic heterogeneity; poor tissue preservation; reinforces its classification as a well-differentiated variant
reliance on tumour cell structure rather than functional of oral SCC with an excellent prognosis and indolent
characteristics; and evaluation of tumour cell features in clinical behaviour.20–30
isolation from those of the surrounding supporting tis- Although the mucous membranes of the head and
sues and cells.4,6,9 neck are the most common sites of VC development,
Furthermore, the growth of the malignant tumour especially the oral cavity and larynx, this tumour may
is frequently accompanied by a dense inflammatory in- also be found on other cutaneous surfaces including
filtrate. A still-unanswered question is whether these the anorectal region, external genitals and skin of the
cells are involved in promoting progression of tumours extremities, particularly the sole of the foot.23,30,31
JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 • 341
––– Oliveira –––
Some investigators have described
“hybrid” lesions, seen when tumours
present the dominant microscopic
features of VC, but also contain small
areas of tumour invasion, which are
common in conventional SCCs.14,36
However, others consider that the
presence of tumour invasion demands
a histopathologic diagnosis of SCC,
because this indicator will dictate
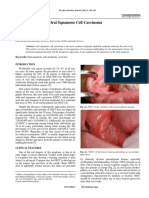
Figure 2: Conventional squamous cell Figure 3: Clinical presentation of clinical treatment.
carcinoma in the floor of the mouth. verrucous carcinoma arising from Although adequate surgical exci-
gingiva. sion remains the treatment of choice
for the oral VC, 24,28,31,37 chemotherapy,
alone or in combination with radio-
therapy, 25,31,37,38 has also been em-
ployed as initial treatment. Radiation
Since Ackerman’s20 original description, there have therapy alone has been contraindicated due to the possi-
been ongoing discussions regarding the etiology of oral bility of anaplastic transformation from oral VC to more
VC. The pathogenesis of this neoplasm has been asso- aggressive SCC. 31,38
ciated with benign verrucous lesions22,31 and tobacco The local recurrence of oral VC has been reported
carcinogenic factors, especially those related to to- frequently. 21,22,31,33,38 Our own retrospective study39 of
bacco chewing. 21,24,28–31 However, there are also reports 3,500 primary oral well-differentiated SCCs surgically ex-
of patients who developed oral VC without a history cised between 1980 and 2002, in which 20 oral VCs were
of smoking.23,26,32 A probable relation between oral VC identified and treated by excision surgery, showed 38.5%
and human papillomavirus has also been suggested. A local recurrence rate. The tumours occurred mainly in
more acceptable hypothesis is that opportunist viral ac- men older than 60; the sites of most common occurrence
tivity associated with chronic tobacco and alcohol con- were the lower lip and the hard palate. Regional recur-
sumption may be involved in the pathogenesis of this rence and distant metastasis were not verified in our
neoplasm. 33–35 sample of oral VCs and all cases presented free surgical
Clinically, VC in the oral cavity is characterized by margins. Based on these results, we suggest that, although
a cauliflower-like exophytic growth with a cleft, warty, it is rare and is associated with an excellent prognosis, VC
whitish-to-gray surface which may have erythematous has an intrinsic potential for local recurrence that should
areas 4,20–24,26,28,29 (Fig. 3). Despite being curable in the be considered when planning surgery.
early stages, this neoplasia can become locally invasive if
it is not treated correctly.20,23,24,31 Basaloid Squamous Cell Carcinoma
Microscopically, VC is a predominantly exophytic Basaloid squamous cell carcinoma (BSCC) is a rare
growth of well-differentiated stratified squamous epi- and aggressive variant of SCC that was first identified as
thelium with deep bulbous rete ridges that exhibit little a separate histopathologic entity by Wain and others.40
or no cytologic atypia and deep surface invaginations This tumour has a predilection for the head and neck
filled with parakeratin or orthokeratin (Fig. 1). The le- region and occurs mainly in the larynx, hypopharynx,
sion’s margins show a compressive growth pattern and oropharynx, epiglottis and at the base of the tongue.4,41
local destruction of connective tissue can occur in ad- Cadier and others 42 first reported it in the oral cavity,
vance of the deep epithelial (“pushing”) border. Despite and isolated lesions have been described in the palate,43,44
exaggerated rete pegs, the associated basement mem- floor of the mouth45 and tuberosity area of the maxilla.46
brane appears intact.4,14,22,23 In the most advanced stages, BSCC is most prevalent among men who are in the sixth
bone, salivary glands, muscles and cartilage involvement or seventh decade of life and have a history of heavy
can be seen. However, distant metastasis24 and regional smoking and alcohol abuse. Clinically, the tumour ap-
lymph node involvement, which occur in conventional pears as an ulcerated, exophytic, firm mass.12,40,47,48
SCC, are rare.4,14 Microscopically, BSCC can have lobular, cord-like,
The establishment of a clinical or histopathologic cribriform, tubular, glandular-like or nest patterns,
diagnosis of VC in the oral cavity may be difficult. 23 It focally connected to the surface epithelium.49 Cells at
depends heavily on close collaboration between clinician the periphery of the lobules are often palisaded, with
and pathologist and the availability of a sufficiently large hyperchromatic nuclei and scant cytoplasm. The central
biopsy specimen.26,27,31,36 areas of the lobules are characterized by cystic spaces,
342 JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 •
––– Subtypes Oral Squamous Cell –––
sometimes containing material resembling mucin, which have not been completely established, probably due to
stains with periodic acid-Schiff, and displaying comedo- the low frequency of these subtypes in the oral cavity. In
necrosis (Fig. 1). The distinctive histologic feature of this addition, many histologic variants of SCC are misdiag-
neoplasm is its appearance as an SCC in intimate relation nosed, either because the biopsy sample is not adequately
with a basaloid component.4,12,40 representative or because of the difficulty of establishing
It is difficult to establish a diagnosis of BSCC in the a diagnosis based on histopathologic features with rou-
head and neck region confidently when only a small tine hematoxylin-eosin staining. Multiple biopsies from
specimen is available for biopsy. 50 The lack of a repre- various areas of the lesion should be obtained to ensure
sentative sample containing a basaloid component as- correct diagnosis of the subtypes.
sociated (or not) with a squamous component or the lack Microscopic identification of the various histologic
of continuity with the epithelium of the oral mucosa subtypes of SCC should be emphasized among patholo-
could lead to diagnosis of poorly differentiated SCC or gists to contribute to consistent knowledge of clinical and
salivary gland tumours, particularly adenoid cystic car- biological tumour behaviour in the oral cavity and result
cinoma.47,51–53 Therefore, when a diagnosis of poorly dif- in more accurate treatment protocols. a
ferentiated SCC is established by incisional biopsy, the
possibility of a BSCC should not be excluded, especially THE AUTHORS
when the lesion is located in the oropharynx or base of
the tongue.
The clinical course and prognosis of BSCC have been Dr. Pereira is a PhD student in the department of stomatology,
considered worse than for conventional SCC based on division of pathology, Bauru School of Dentistry, University of
São Paulo, Brazil.
its aggressive biological behaviour characterized by early
local or regional recurrences and distant metastasis, as
well as lower reported survival rates.41,46,49–52 However, few Dr. Oliveira is associate professor in the department of sto-
studies have compared the clinical course and biological matology, division of pathology, Bauru School of Dentistry,
behaviour of oral BSCC and SCC50,51,54 and sample sizes University of São Paulo, Brazil.
are too small to be representative or allow the establish-
ment of a prognosis.
In a review of BSCCs affecting only the oral cavity, Dr. Landman is a pathologist in the department of pathology,
A.C. Camargo Cancer Hospital, São Paulo, Brazil.
Sampaio-Goes and others12,48 compared 17 BSCCs with
well, moderately and poorly differentiated SCCs, matched
by stage and site, in terms of clinical and biologic course,
outcome, treatment and prognosis. The authors concluded Dr. Kowalski is director, department of head and neck surgery
and otorhinolaryngology, A.C. Camargo Cancer Hospital,
that the clinical and biological course of BSCC is similar to São Paulo, Brazil.
that of SCC when clinical stage and site are matched. Thus,
they suggest that patients with these malignant neoplasms
Correspondence to: Dr. Denise Tostes Oliveira, Department of
undergo the same therapeutic protocols as for SCC. Stomatology, Division of Pathology, Bauru School of Dentistry, Alameda
Octávio Pinheiro Brisolla, 9-75 CEP 17012-901, Bauru, São Paulo, Brazil.
Other Histologic Variants of Squamous Cell The authors have no declared financial interests.
Carcinoma
Several subtypes of oral SCC, including spindle-cell This article has been peer reviewed.
carcinoma, papillary SCC, adenosquamous carcinoma,
acantholytic SCC and carcinoma cuniculatum, were con- References
1. Scully C, Newman L, Bagan JV. The role of the dental team in preventing
sidered in the classification adopted by the IARC–WHO.14 and diagnosing cancer: 2. Oral cancer risk factors. Dent Update 2005;
Most described cases of these malignant neoplasms have 32(5):261–2, 264–6, 269–70.
2. Mignogna MD, Fedele S, Lo Russo L. The World Cancer Report and the
been in the larynx and hypopharynx. burden of oral cancer. Eur J Cancer Prev 2004; 13(2):139–42.
Due to the small number of reported cases of these 3. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J
histologic subtypes in the oral cavity,11,39 information Clin 2002; 52(4):195–215.
about their prognosis and clinical–biological behaviour 4. Pindborg JJ, Reichart PA, Smith CJ, van der Wall I. Histological typing of
cancer and precancer of the oral mucosa. 2nd ed. Berlin: Springer-Verlag;
has not been established.4,14 1997. p. 10–6.
5. Bankfalvi A, Piffko J. Prognostic and predictive factors in oral cancer: the
role of the invasive tumour front. J Oral Pathol Med 2000; 29(7):291–8.
Conclusion 6. Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, and others.
Although SCC is the most frequent malignant neo- Prognostic factors of clinically stage I and II oral tongue carcinoma — a
comparative study of stage, thickness, shape, growth pattern, invasive front
plasm of the oral cavity, its clinical and biological course malignancy grading, Martinez-Gimeno score, and pathologic features. Head
and the prognosis associated with its histologic variants Neck 2002; 24(6):513–20.
JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 • 343
––– Oliveira –––
7. Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. 32. Link JO, Kaugars GE, Burns JC. Comparison of oral carcinomas in smoke-
Tumor-associated tissue eosinophilia as a prognostic factor in oral squamous less tobacco users and nonusers. J Oral Maxillofac Surg 1992; 50(5):452–5.
cell carcinoma. Histopathology 2002; 41(2):152–7. 33. Eisenberg E, Rosenberg B, Krutchkoff DJ. Verrucous carcinoma: a pos-
8. Karakida K, Ota Y, Aoki T, Yamazaki H, Tsukinoki K. Examination of fac- sible viral pathogenesis. Oral Surg Oral Med Oral Pathol 1985; 59(1):52–7.
tors predicting occult metastasis of the cervical lymph nodes in T1 and T2
34. Adler-Storthz K, Newland JR, Tessin BA, Yeudall WA, Shillitoe EJ. Human
tongue carcinoma. Tokai J Exp Clin Med 2002; 27(3):65–71.
papillomavirus type 2 DNA in oral verrucous carcinoma. J Oral Pathol 1986;
9. O-charoenrat P, Pillai G, Patel S, Fisher C, Archer D, Eccles S, and other.
15(9):472–5.
Tumour thickness predicts cervical nodal metastases and survival in early oral
tongue cancer. Oral Oncol 2003; 39(4):386–90. 35. Saito T, Nakajima T, Mogi K. Immunohistochemical analysis of cell
10. Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tomoyose T, Tanaka cycle-associated proteins p16, pRb, p53, p27 and Ki-67 in oral cancer and
T, and others. The high prognostic value of the histologic grade at the deep precancer with special reference to verrucous carcinomas. J Oral Pathol Med
invasive front of tongue squamous cell carcinoma. J Oral Pathol Med 2005; 1999; 28(5):226–32.
34(6):329–33. 36. Niparko JK, Rubinstein MI, McClatchey KD. Invasive squamous cell car-
11. Woolgar JA. Histopathological prognosticators in oral and oropharyn- cinoma within verrucous carcinoma. J Otolaryngol 1988; 17(1):38–40.
geal squamous cell carcinoma. Oral Oncol 2006; 42(3):229–39. Epub 2005 37. Pomatto E, Bocca M, Carbone V, Vercellino V. [Verrucous carcinoma of
Sep 16. the oral cavity. Personal experience with combined chemo-surgical-treat-
12. Sampaio-Goes FC, Oliveira DT, Dorta RG, Nonogaki S, Landman G, ment]. [Article in Italian] Minerva Chir 1993; 48(5):213–9.
Nishimoto IN, and other. Expression of PCNA, p53, Bax and Bcl-X in oral 38. Yoshimura Y, Mishima K, Obara S, Nariai Y, Yoshimura H, Mikami T.
poorly differentiated and basaloid squamous cell carcinoma: relationships
Treatment modalities for oral verrucous carcinomas and their outcomes:
with prognosis. Head Neck 2005; 27(11):982–9.
contribution of radiotherapy and chemotherapy. Int J Clin Oncol 2001;
13. Heffner DK. Let’s make grading of squamous cell carcinomas more
6(4):192–200.
meaningful to clinicians (via “Ed’s Insight”). Ann Diagn Pathol 2002;
6(6):399–403. 39. Oliveira DT, Moraes RV, Fiamengui Filho J, Neto JF, Landman G, Kowalski
14. Barnes L, Eveson JW, Reichart P, Sidransky D. Oral cavity and oropharynx. LP. Oral verrucous carcinoma: a retrospective study in São Paulo Region,
In: Barnes L, Everson JW, Reichart P, Sidransky D, editors. World Health Brazil. Clin Oral Investig 2006; 10(3):205–9
Organization classification of tumours. Pathology and genetics of head and 40. Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of
neck tumours. Lyon: International Agency for Research on Cancer Press; the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol 1986;
2005. p. 164–208. 17(11):1158–66.
15. Johnson N, Franceschi S, Ferlay J, Ramadas K, Schmid S, MacDonald DG, 41. Klijanienko J, el-Naggar A, Ponzio-Prion A, Marandas P, Micheau
and others. Squamous cell carcinoma. In: Barnes L, Everson JW, Reichart P, C, Caillaud JM. Basaloid squamous carcinoma of the head and neck.
Sidransky D, editors. World Health Organization classification of tumours. Immunohistochemical comparison with adenoid cystic carcinoma and
Pathology and genetics of head and neck tumours. Lyon: International squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 1993;
Agency for Research on Cancer Press; 2005. p. 168–75.
119(8):887–90.
16. Strong EW, Kasdorf H, Henk JM. Squamous cell carcinoma of the head
42. Cadier MA, Kelly SA, Parkhouse N, Brough MD. Basaloid squamous car-
and neck. In: Hermanek P, Gospodarowicz MK, Henson DE, Hutter RV,
Sobin LH, editors. Prognostic factors in cancer. New York: Springer; 1996. cinoma of the buccal cavity. Head Neck 1992; 14(5):387–91.
p. 13–20. 43. Lovejoy HM, Matthews BL. Basaloid-squamous carcinoma of the palate.
17. Bundgaard T, Rossen K, Henriksen SD, Charabi S, Sogaard H, Grau C. Otolaryngol Head Neck Surg 1992; 106(2):159–62.
Histopathologic parameters in the evaluation of T1 squamous cell carcin- 44. Hellquist HB, Dahl F, Karlsson MG, Nilsson C. Basaloid squamous cell
omas of the oral cavity. Head Neck 2002; 24(7):656–60. carcinoma of the palate. Histopathology 1994; 25(2):178–80.
18. Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against 45. Campman SC, Gandour-Edwards RF, Sykes JM. Basaloid squamous car-
cancer. Immunol Lett 2003; 90(2-3):103–22. cinoma of the head and neck. Report of a case occurring in the anterior floor
19. Gonzalez-Moles MA, Galindo P, Gutierrez-Fernandez J, Sanchez- of the mouth. Arch Pathol Lab Med 1994; 118(12):1229–32.
Fernandez E, Rodriguez-Archilla A, Ruiz-Avila I, and other. P53 protein 46. Wedenberg C, Jesslen P, Lundqvist G, Lundgren J, Hellquist HB. Basaloid
expression in oral squamous cell carcinoma: survival analysis. Anticancer Res
squamous cell carcinoma of the maxilla. Oral Oncol 1997; 33(2):141–4.
2001; 21(4B):2889–94.
47. Altavilla G, Mannara GM, Rinaldo A, Ferlito A. Basaloid squamous cell
20. Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery 1948;
23:670–8. carcinoma of the oral cavity and oropharynx. ORL J Otorhinolaryngol Relat
Spec 1999; 61(3):169–73.
21. Kraus F, Perezmesa C. Verrucous carcinoma. Clinical and pathologic
study of 105 cases involving oral cavity, larynx and genitalia. Cancer 1966; 48. de Sampaio Goes FC, Oliveira DT, Dorta RG, Nishimoto IN, Landman
19(1):26–38. G, Kowalski LP. Prognoses of oral basaloid squamous cell carcinoma and
22. Jacobson S, Shear M. Verrucous carcinoma of the mouth. J Oral Pathol squamous cell carcinoma: a comparison. Arch Otolaryngol Head Neck Surg
1972; 1(2):66–75. 2004; 130(1):83–6.
23. McCoy JM, Waldron CA. Verrucous carcinoma of the oral cavity. A review 49. Banks ER, Frierson HF Jr, Mills SE, George E, Zarbo RJ, Swanson PE.
of forty-nine cases. Oral Surg Oral Med Oral Pathol 1981; 52(6):623–9. Basaloid squamous cell carcinoma of the head and neck. A clinicopatho-
24. Rajendran R, Sugathan CK, Augustine J, Vasudevan DM, Vijayakumar T. logic and immunohistochemical study of 40 cases. Am J Surg Pathol 1992;
Ackerman’s tumour (Verrucous carcinoma) of the oral cavity: a histopatho- 16(10):939–46.
logic study of 426 cases. Singapore Dent J 1989; 14(1):48–53. 50. Abiko Y, Muramatsu T, Tanaka Y, Ohuchi T, Satoh M, Okumura K, and
25. Jyothirmayi R, Sankaranarayanan R, Varghese C, Jacob R, Nair MK. others. Basaloid-squamous cell carcinoma of the oral mucosa: report of two
Radiotherapy in the treatment of verrucous carcinoma of the oral cavity. Oral cases and study of the proliferative activity. Pathol Int 1998; 48(6):460–6.
Oncol 1997; 33(2):124–8. 51. Coppola D, Catalano E, Tang CK, Elfenbein IB, Harwick R, Mohr R.
26. Bouquot JE. Oral verrucous carcinoma. Incidence in two US populations. Basaloid squamous cell carcinoma of floor of the mouth. Cancer 1993;
Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 86(3):318–24. 72(8):2299–305.
27. Ferlito A, Rinaldo A, Mannara GM. Is primary radiotherapy an appro-
52. Raslan WF, Barnes L, Krause JR, Contis L, Killeen R, Kapadia SB. Basaloid
priate option for the treatment of verrucous carcinoma of the head and
squamous cell carcinoma of the head and neck: a clinicopathologic and flow
neck? J Laryngol Otol 1998; 112(2):132–9.
cytometric study of 10 new cases with review of English literature. Am J
28. Spiro RH. Verrucous carcinoma, then and now. Am J Surg 1998;
Otolaryngol 1994; 15(3):204–11.
176(5):393–7.
53. Barnes L, Ferlito A, Altavilla G, MacMillan C, Rinaldo A, Doglioni C.
29. Kaugars GE, Abbey LM, Burns JC, Page DG, Svirsky JA. Oral verru-
cous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; Basaloid squamous cell carcinoma of the head and neck: clinicopatho-
87(3):268–9. logical features and differential diagnosis. Ann Otol Rhinol Laryngol 1996;
30. Chagin AL. Factores de riesgo etiopatogenicos del carcinoma verrugoso 105(1):75–82.
en cavidad bucal. Acta Odontol Venez 2000; 38(2):9–14. 54. Coletta RD, Cotrim P, Vargas PA, Villalba H, Pires FR, de Moraes M, and
31. Koch BB, Trask DK, Hoffman HT, Karnell LH, Robinson RA, Zhen W, and other. Basaloid squamous carcinoma of the oral cavity: report of 2 cases and
others. National survey of head and neck verrucous carcinoma: patterns of study of AgNOR, PCNA, p53, and MMP expression. Oral Surg Oral Med Oral
presentation, care, and outcome. Cancer 2001; 92(1):110–20. Pathol Oral Radiol Endod 2001; 91(5):563–9.
344 JCDA • www.cda-adc.ca/jcda • May 2007, Vol. 73, No. 4 •
View publication stats
You might also like
- Apogee 1000 Neo Data Sheet - V2.X - 180212Document10 pagesApogee 1000 Neo Data Sheet - V2.X - 180212IBrahim FayedNo ratings yet
- Glorasi Anatomi - Arnold GlosaryDocument29 pagesGlorasi Anatomi - Arnold GlosaryRoszita TamyizNo ratings yet
- Articulo 1Document17 pagesArticulo 1Andrea NeriaNo ratings yet
- Journal Second Primary OSCCDocument8 pagesJournal Second Primary OSCCJames BondNo ratings yet
- PIIS2212440318300920Document12 pagesPIIS2212440318300920EmiliaAndreeaBalanNo ratings yet
- Deepak Kademani - Improving Outcomes in Oral Cancer - A Clinical and Translational Update-Springer International Publishing (2020)Document189 pagesDeepak Kademani - Improving Outcomes in Oral Cancer - A Clinical and Translational Update-Springer International Publishing (2020)Shantanu DixitNo ratings yet
- Clear Cell Carcinoma of TongueDocument7 pagesClear Cell Carcinoma of TongueMadhura ShekatkarNo ratings yet
- Bjc2011403a PDFDocument11 pagesBjc2011403a PDFPragatiNo ratings yet
- JOMFP 19 267bDocument4 pagesJOMFP 19 267bThái BìnhNo ratings yet
- 2014 - Cannibalism - Full ArticleDocument6 pages2014 - Cannibalism - Full ArticleUma DatarNo ratings yet
- Ejd - Ejd 261 16Document5 pagesEjd - Ejd 261 16Neggy YudibrataNo ratings yet
- Squamous Cell Carcinoma of The Gingiva: A Case Report and Literature ReviewDocument6 pagesSquamous Cell Carcinoma of The Gingiva: A Case Report and Literature ReviewBayuNo ratings yet
- Histological and Molecular Aspects of Oral Squamous Cell Carcinoma (Review)Document5 pagesHistological and Molecular Aspects of Oral Squamous Cell Carcinoma (Review)Yeni PuspitasariNo ratings yet
- Perspectives On Oral Squamous Cell Carcinoma Prevention - Proliferation, Position, Progression and PredictionDocument14 pagesPerspectives On Oral Squamous Cell Carcinoma Prevention - Proliferation, Position, Progression and PredictionFannyNo ratings yet
- Velscope: Shedding Light On Its Ideal Application: AcronymsDocument7 pagesVelscope: Shedding Light On Its Ideal Application: AcronymsSaraswita Gabriellah SaetikhoNo ratings yet
- Salivary Gland TumorsDocument13 pagesSalivary Gland TumorsHernan JJNo ratings yet
- CarcinogenesisDocument7 pagesCarcinogenesisTejas KulkarniNo ratings yet
- Glandular Odontogenic Cyst Analysis of 46 CasesDocument12 pagesGlandular Odontogenic Cyst Analysis of 46 CasesSenussi IbtisamNo ratings yet
- Invasive Squamous Cell Carcinoma: Comparison of Differentiation Grade and Tumour Depth by Anatomical Site in 1666 TumoursDocument8 pagesInvasive Squamous Cell Carcinoma: Comparison of Differentiation Grade and Tumour Depth by Anatomical Site in 1666 TumoursEstefy Cerda MoscosoNo ratings yet
- Hed 44 1481Document11 pagesHed 44 1481dayllyn iglesiasNo ratings yet
- Better Grade of Tumor Differentiation of Oral Squamous Cell Carcinoma Arising in Background of Oral Submucous FibrosisDocument5 pagesBetter Grade of Tumor Differentiation of Oral Squamous Cell Carcinoma Arising in Background of Oral Submucous FibrosisDIVYABOSENo ratings yet
- Review Article: Keratocystic Odontogenic Tumor (Parakeratinised Oodontogenic Keratocyst) - A ReviewDocument6 pagesReview Article: Keratocystic Odontogenic Tumor (Parakeratinised Oodontogenic Keratocyst) - A ReviewElijah JoeNo ratings yet
- Oral Cavity and Lip Cancer: United Kingdom National Multidisciplinary GuidelinesDocument7 pagesOral Cavity and Lip Cancer: United Kingdom National Multidisciplinary Guidelineskepipranave-9084No ratings yet
- Haake Et Al 2017 CancerDocument10 pagesHaake Et Al 2017 Cancerdiego peinadoNo ratings yet
- Head Neck - 2023 - Weaver - A Molecular Guide To Systemic Therapy in Salivary Gland CarcinomaDocument12 pagesHead Neck - 2023 - Weaver - A Molecular Guide To Systemic Therapy in Salivary Gland CarcinomaafissaNo ratings yet
- Oral Cancer-GeneticsDocument18 pagesOral Cancer-GeneticsAlberto FilhoNo ratings yet
- DownloadDocument6 pagesDownloadpaulo ricardoNo ratings yet
- Ductal Carcinoma in Situ of Breast: Update 2019: S S. B, Y G - PDocument7 pagesDuctal Carcinoma in Situ of Breast: Update 2019: S S. B, Y G - PAaslesha JakkampudiNo ratings yet
- The Identi Fications and Clinical Implications of Cancer Stem Cells in Colorectal CancerDocument10 pagesThe Identi Fications and Clinical Implications of Cancer Stem Cells in Colorectal CancerManuel GuachoNo ratings yet
- Journal of Dental Research: The Odontogenic Keratocyst: A Cyst, or A Cystic Neoplasm?Document11 pagesJournal of Dental Research: The Odontogenic Keratocyst: A Cyst, or A Cystic Neoplasm?cedo7No ratings yet
- The Management of Aggressive Cysts of The JawsDocument12 pagesThe Management of Aggressive Cysts of The JawsChristian NguyễnNo ratings yet
- Modelos de Raton Con SCC. IshidaDocument5 pagesModelos de Raton Con SCC. IshidaPatrimonio Tecnológico S.L.No ratings yet
- Second Primary OSCC 222Document2 pagesSecond Primary OSCC 222James BondNo ratings yet
- Oral Leukoplakia Remains A Challenging Condition: A Villa - S SonisDocument5 pagesOral Leukoplakia Remains A Challenging Condition: A Villa - S SonisDaniela Suarez TamayoNo ratings yet
- Jurnal OMDocument8 pagesJurnal OMSaraswita Gabriellah SaetikhoNo ratings yet
- Who Is in and Who Is OutDocument6 pagesWho Is in and Who Is OutJuan Carlos MeloNo ratings yet
- Salivary Biomarkers in The Detection of Oral Cancer. 07 OCT BienDocument5 pagesSalivary Biomarkers in The Detection of Oral Cancer. 07 OCT BienKevin FossNo ratings yet
- Articulo 9Document11 pagesArticulo 9Lucero BallesterosNo ratings yet
- Oral Cavity CancerDocument13 pagesOral Cavity CancerLuthfianaNo ratings yet
- 95 123 3 PBDocument6 pages95 123 3 PBNeggy YudibrataNo ratings yet
- 1 Tmu - JD - 039 PDFDocument4 pages1 Tmu - JD - 039 PDFKristina SabuNo ratings yet
- 1 Tmu - JD - 039Document4 pages1 Tmu - JD - 039Hasan BlackNo ratings yet
- Update On Nasopharyngeal Carcinoma: Ó Humana 2007Document6 pagesUpdate On Nasopharyngeal Carcinoma: Ó Humana 2007Zakia AjaNo ratings yet
- W de WenisimoDocument18 pagesW de WenisimoBJ CarminatorNo ratings yet
- Assessment of Clinical Examination Validity in Oral Cancer Risk PatientsDocument7 pagesAssessment of Clinical Examination Validity in Oral Cancer Risk PatientsVera Radojkova NikolovskaNo ratings yet
- Keratoacanthoma (KA) : An Update and Review: EviewsDocument14 pagesKeratoacanthoma (KA) : An Update and Review: EviewsAlejandraGonzalezRodriguezNo ratings yet
- 2009 0527 rs.1 PDFDocument5 pages2009 0527 rs.1 PDFpaolaNo ratings yet
- Velscope Oral PremalignancyDocument5 pagesVelscope Oral PremalignancySusanaSanoNo ratings yet
- QueratoacantomaDocument14 pagesQueratoacantomaAntonieta NumaNo ratings yet
- Todentj 6 126Document5 pagesTodentj 6 126AngelicaNo ratings yet
- Current Aspects On Oral Squamous Cell Carcinoma: Anastasios K. MarkopoulosDocument5 pagesCurrent Aspects On Oral Squamous Cell Carcinoma: Anastasios K. MarkopoulosYeni PuspitasariNo ratings yet
- Safari - 17 Okt 2019 05.51Document1 pageSafari - 17 Okt 2019 05.51dian siswojoNo ratings yet
- Assessment of Apoptotic Index in Oral Epithelial Dysplasia and Oral Squamous Cell CarcinomaDocument3 pagesAssessment of Apoptotic Index in Oral Epithelial Dysplasia and Oral Squamous Cell CarcinomaMadhura ShekatkarNo ratings yet
- Oral Field CancerizationDocument7 pagesOral Field CancerizationFarah UlyaNo ratings yet
- Routray 2013 PDFDocument4 pagesRoutray 2013 PDFDrMohit SharmaNo ratings yet
- Histological, Molecular and Functional SubtypesDocument7 pagesHistological, Molecular and Functional SubtypesCristopherJairEstupiñanVirúNo ratings yet
- OrthoTrauma - 2017 - Beckingsale - Epidemiology of Bone and ST SarcomasDocument7 pagesOrthoTrauma - 2017 - Beckingsale - Epidemiology of Bone and ST SarcomasJacob DoughertyNo ratings yet
- MixomaDocument7 pagesMixomaNICOLENo ratings yet
- Oral Oncology: Juan Seoane Lestón, Pedro Diz DiosDocument5 pagesOral Oncology: Juan Seoane Lestón, Pedro Diz DiosSULTAN1055No ratings yet
- Spatial Transcriptomics Reveals Distinct and Conserved Tumor Core and Edge Architectures That Predict Survival and Targeted Therapy ResponseDocument61 pagesSpatial Transcriptomics Reveals Distinct and Conserved Tumor Core and Edge Architectures That Predict Survival and Targeted Therapy Response戴义宾No ratings yet
- Oral Squamous Cell Carcinoma Epidemiology ClinicalDocument7 pagesOral Squamous Cell Carcinoma Epidemiology Clinicalhtpxol1No ratings yet
- Theoretical Questions - Head and NeckDocument3 pagesTheoretical Questions - Head and NeckImtiaz SalahaniNo ratings yet
- Bio7 ch48Document20 pagesBio7 ch48freezo1994No ratings yet
- Frog Embryology PDFDocument17 pagesFrog Embryology PDFHohai ConcepcionNo ratings yet
- Process of DigestionDocument4 pagesProcess of DigestionKennard LIONGNo ratings yet
- Ncma217 Lec PrelimDocument38 pagesNcma217 Lec PrelimIVY SUPNETNo ratings yet
- Animal Kingdom-Class 11Document6 pagesAnimal Kingdom-Class 11ICSE BOARDNo ratings yet
- Cambridge IGCSE: Biology 0610/21Document16 pagesCambridge IGCSE: Biology 0610/21Miranda KwendambairiNo ratings yet
- Jurnal Mengkudu InggrisDocument10 pagesJurnal Mengkudu Inggrisfebryanti 29No ratings yet
- Checkpoint Science Revision GuideDocument252 pagesCheckpoint Science Revision Guidemixiaoquan98No ratings yet
- Anthoceros ANTHOCEROS (Hornwort) : Morphology of ThallusDocument3 pagesAnthoceros ANTHOCEROS (Hornwort) : Morphology of ThallusyeateshwarriorNo ratings yet
- Full Download Test Bank For Human Physiology 15th Edition by Stuart Fox PDF Full ChapterDocument36 pagesFull Download Test Bank For Human Physiology 15th Edition by Stuart Fox PDF Full Chapterduringsclaverv8a100% (18)
- Intermediate Guide To Aerial SilkDocument139 pagesIntermediate Guide To Aerial Silkffbd9fpkrhNo ratings yet
- Tabel Perbandingan MSC Dan EksosomDocument5 pagesTabel Perbandingan MSC Dan EksosomRaisha FathimaNo ratings yet
- Manual Nervous SystemDocument6 pagesManual Nervous SystemVynz Morales CosepNo ratings yet
- 1 SM PDFDocument7 pages1 SM PDFandini fitriNo ratings yet
- A Newly Married Couple Does Not Want Have Children For Few YearsDocument6 pagesA Newly Married Couple Does Not Want Have Children For Few YearsSHILPA AGARWALNo ratings yet
- Four Chambers of The HeartDocument7 pagesFour Chambers of The HeartJohn TecsonNo ratings yet
- Leukocytes 2Document19 pagesLeukocytes 2Tabitha EaglehouseNo ratings yet
- Power Point EmgDocument39 pagesPower Point EmgsridharNo ratings yet
- Spots For Hematology PracticalDocument17 pagesSpots For Hematology PracticalPhysiology by Dr RaghuveerNo ratings yet
- Barret's & Tumors of Esophagus, SR, April 20Document52 pagesBarret's & Tumors of Esophagus, SR, April 20Tehreem NadeemNo ratings yet
- Jaringan Meristem Dan Jeringan PermanenDocument4 pagesJaringan Meristem Dan Jeringan PermanenAulia FitriNo ratings yet
- Benign Ovarian TumourDocument45 pagesBenign Ovarian Tumourahmed shorshNo ratings yet
- Musculoskeletal SystemDocument22 pagesMusculoskeletal SystemKeyna DizonNo ratings yet
- Grade 12 Cells Review - QuizizzDocument10 pagesGrade 12 Cells Review - QuizizzkyisinlinlettNo ratings yet
- SGD Case Number 3Document7 pagesSGD Case Number 3kris angNo ratings yet
- PyelonephritisDocument59 pagesPyelonephritisGheyl GabrielNo ratings yet
- Movement of Substances Across Membrane - Public Exam Questions (Answer Keys)Document4 pagesMovement of Substances Across Membrane - Public Exam Questions (Answer Keys)Henry LamNo ratings yet