Professional Documents
Culture Documents
General Organic Chemistry (GOC) - DPP 01 - Removed
Uploaded by
shishiranand250 ratings0% found this document useful (0 votes)
3 views2 pagesOriginal Title
General Organic Chemistry(GOC) _ DPP 01_removed
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pagesGeneral Organic Chemistry (GOC) - DPP 01 - Removed
Uploaded by
shishiranand25Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
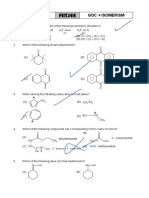
1
ARJUNA (JEE)
DPP-01
General Organic Chemistry
1. The inductive effect of the groups: –NH3; 6. Order of stability of given free radical is:
–D; –CO2Θ; –COOH are respectively H3 C CH 2 H3C C H CH3
(A) –I, +I, +I, –I (I) (II)
(B) –I, –I, –I, +I H 3 C C CH 2 CH 3
(C) +I, No effect, –I, –I |
(D) +I, –I, –I, –I CH 3
(III)
(A) III > I > II (B) I > II > III
2. Which of the following belongs to +I (C) II > I > III (D) III > II > I
group?
(A) –OMe (B) NH 3 7. Arrange the following groups in the order
of decreasing (–I) effect.
O
|| (A) CN > F > Br > Cl > COOH > I > H
(C) C O (D) –OH (B) COOH > CN > F > Br > Cl > I > H
(C) H > COOH > CN > I > Cl > F > Cl
(D) CN > COOH > F > Cl > Br > I > H
3. Choose the correct statement
(A) I effect operates in both and bonds 8. The +I.E. (inductive effect) is shown by:
(B) I effect creates net charge in molecule (A) –CH3 (B) –OH
(C) I effect transfers electron from one (C) –F (D) –C6H5
carbon to another
(D) I effect creates partial charges and it is 9. The –I effect is shown by:
distance dependent (A) –COOH (B) –CH3
(C) –CH3CH2 (D) –CHR2
4. The most stable carbocation among the
following is: 10. The increasing order of +ve I-effect shown
by H, CH3, C2H5 and C3H7 is:
(A) H3C (A) H < CH3 < C2H5 < C3H7
(B) H > CH3 > C2H5 >C3H7
(B) H 2 C CH 2 OCH 3
(C) H < C2H5 < CH3 < C3H7
(C) H 3 C C CH 2 CH 3 (D) None of the above
|
CH 3 11. The most acidic hydrogen among given
compounds is:
(D) H 3 C C CH 2 OCH 3 Ha
| |
CH 3 (A) H3C H 2 C CH 2
Ha
5. The increase in number of alkyl groups |
(A) Increases the stability of carbanion (B) H3C CH 2 CH CH3
Ha
(B) Decreases the stability of carbanion |
(C) Remains the same (C) H 3 C C CH 3
(D) None of these |
CH 3
(D) Cannot be predicted
2
12. The most acidic compound among
following is:
(A) F – CH2 – COOH
(B) Cl – CH2 – COOH
(C) Br – CH2 – COOH
(D) I – CH2 – COOH
You might also like
- General Organic Chemistry - DPP 01Document3 pagesGeneral Organic Chemistry - DPP 01suryawanshiashish549No ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Halogen Derivatives (13th)Document31 pagesHalogen Derivatives (13th)Raju SinghNo ratings yet
- Goc 6002eec9ca05fDocument14 pagesGoc 6002eec9ca05fAyush TiwariNo ratings yet
- Gocdpp 60Document8 pagesGocdpp 60Mrigank GuptaNo ratings yet
- Diwali Assignment 13thDocument8 pagesDiwali Assignment 13thRaju SinghNo ratings yet
- GOC DPP 1 - StudentDocument3 pagesGOC DPP 1 - StudentVanshdip RawatNo ratings yet
- GOC (13th)Document34 pagesGOC (13th)Raju SinghNo ratings yet
- GOC DPPDocument35 pagesGOC DPPRayNo ratings yet
- CPP (GOC-2) RBooster ResDocument10 pagesCPP (GOC-2) RBooster RessahilNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- ProjectDocument17 pagesProjectrudrashah4777No ratings yet
- Organic Chemistry: Daily Practice ProblemsDocument10 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Research PaperDocument16 pagesResearch PaperShounak DuttaNo ratings yet
- Basic Concept in Org. Chem - Exercise-Eng Module-4Document25 pagesBasic Concept in Org. Chem - Exercise-Eng Module-4Raju SinghNo ratings yet
- General Organic Chemistry Practice SheetDocument7 pagesGeneral Organic Chemistry Practice Sheetrahul kmNo ratings yet
- Practice Paper - Phase 3Document6 pagesPractice Paper - Phase 3Dhairya VinayakNo ratings yet
- OrganicDocument9 pagesOrganicjitesh100kushwahaNo ratings yet
- Organic Chemistry: Daily Practice ProblemsDocument53 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Goc-II Ex e SiamrpnDocument22 pagesGoc-II Ex e SiamrpnArchit KhemaniNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- GOC ExerciseDocument45 pagesGOC ExerciseAMAR DEEP SHUKLA100% (3)
- Or-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WADocument23 pagesOr-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WAUppu EshwarNo ratings yet
- Part - I: Objective Questions: Section A: Geometrical IsomerismDocument10 pagesPart - I: Objective Questions: Section A: Geometrical IsomerismTejas pawarNo ratings yet
- 1.2 Isomerism AssignmentDocument10 pages1.2 Isomerism AssignmentTejas pawarNo ratings yet
- GocDocument20 pagesGocSuyog SardaNo ratings yet
- Guided Plan-5 (E)Document4 pagesGuided Plan-5 (E)abhiraw30062005No ratings yet
- Carbonyl CompoundsDocument14 pagesCarbonyl CompoundsSunderNo ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- Day - 28 Goc-2 (27-12-2022) ChemistryDocument4 pagesDay - 28 Goc-2 (27-12-2022) ChemistryVIKRANTH KUMAR JAKKOJUNo ratings yet
- First Year - GOC, ISOMERISM - Revision - CPP - CKHDocument3 pagesFirst Year - GOC, ISOMERISM - Revision - CPP - CKHSeaN GabrielNo ratings yet
- 13DPP25FGOCEXCELMIXEDDocument5 pages13DPP25FGOCEXCELMIXEDRIP- PIRNo ratings yet
- Alkene DPPDocument20 pagesAlkene DPPKalyan ReddtNo ratings yet
- GOC & EAS CPP-II - PMDDocument14 pagesGOC & EAS CPP-II - PMDVansh sareenNo ratings yet
- Haloalkanes: Target Iit-JeeDocument44 pagesHaloalkanes: Target Iit-JeeHarsh VardhanNo ratings yet
- Goc & Eas Test-IiDocument7 pagesGoc & Eas Test-IiAniket GuptaNo ratings yet
- GujCET - D26 Mar 2023Document34 pagesGujCET - D26 Mar 2023aadityabhagchandaniNo ratings yet
- DPP GoccccDocument10 pagesDPP GoccccMayur Khichi0% (1)
- Clip HydrocarbonDocument29 pagesClip HydrocarbonDhairya VinayakNo ratings yet
- OH OH : Exercise-IDocument12 pagesOH OH : Exercise-IVandana ReddyNo ratings yet
- Organic QuestionsDocument51 pagesOrganic Questionshemab30851No ratings yet
- Exercise 12Document29 pagesExercise 12Soumya AgrawalNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- Carboxylic Acids and It's Derivative Aliphatic AminesDocument32 pagesCarboxylic Acids and It's Derivative Aliphatic AminesRaju SinghNo ratings yet
- Bakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveDocument3 pagesBakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveRitesh SonawaneNo ratings yet
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Guided Plan-6 (E)Document7 pagesGuided Plan-6 (E)abhiraw30062005No ratings yet
- Sheet - 02 - General Organic ChemistryDocument74 pagesSheet - 02 - General Organic ChemistrykeshavNo ratings yet
- Sample Acs Final ExamDocument27 pagesSample Acs Final Examjilo100% (2)
- Alkyl Halide: Organic ChemistryDocument56 pagesAlkyl Halide: Organic ChemistryAaryan KeshanNo ratings yet
- Goc Sheet-3-EfDocument6 pagesGoc Sheet-3-EfAaRaV KuShWaHaNo ratings yet
- Me Me CL BR CH-CH CH Oh: O PCLDocument3 pagesMe Me CL BR CH-CH CH Oh: O PCLAkhil JamwalNo ratings yet
- GOC Rev Ex 6Document3 pagesGOC Rev Ex 6mitsuhaNo ratings yet
- Q.paper Aiims 2021Document190 pagesQ.paper Aiims 2021anandramNo ratings yet
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- Date Planned: - / - / - Daily Tutorial Sheet-9 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-2 Exact DurationDocument1 pageDate Planned: - / - / - Daily Tutorial Sheet-9 Expected Duration: 90 Min Actual Date of Attempt: - / - / - Level-2 Exact DurationVIDYA SRI GANESHNo ratings yet
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Zone Structure ListDocument2 pagesZone Structure Listshishiranand25No ratings yet
- 2-C-2 Eng Lang. and LiteratureDocument8 pages2-C-2 Eng Lang. and Literatureshishiranand25No ratings yet
- ALL SOUTH PREMEDICAL NURTURE MAJOR TEST COMMON FOR ALLPHIII 181688 TEST SOL QRfiGqw3PPDocument10 pagesALL SOUTH PREMEDICAL NURTURE MAJOR TEST COMMON FOR ALLPHIII 181688 TEST SOL QRfiGqw3PPshishiranand25No ratings yet
- Sequence and Series - DPP 09Document2 pagesSequence and Series - DPP 09shishiranand25No ratings yet
- Ilovepdf MergedDocument7 pagesIlovepdf Mergedshishiranand25No ratings yet
- Interactions Between Base Paper and Coating Color in Metered Size Press CoatingDocument99 pagesInteractions Between Base Paper and Coating Color in Metered Size Press CoatingHuy NguyenNo ratings yet
- Concrete Making MaterialsDocument55 pagesConcrete Making Materialsjaffna100% (1)
- Basic Tools of Analytical Chemistry: Chapter OverviewDocument28 pagesBasic Tools of Analytical Chemistry: Chapter OverviewReza LaurinaNo ratings yet
- Homi BhabhaDocument19 pagesHomi BhabhaDeepak GuptaNo ratings yet
- Jurnal 2 Analisis KarbohidratDocument7 pagesJurnal 2 Analisis KarbohidratKeziaNo ratings yet
- Physica E: Qianhui Yang, Liren Lou, Guanzhong WangDocument6 pagesPhysica E: Qianhui Yang, Liren Lou, Guanzhong WangHabibi SaifuddinNo ratings yet
- Review of Cansolv SO Scrubbing System's First Commercial Operations in The Oil Refining IndustryDocument17 pagesReview of Cansolv SO Scrubbing System's First Commercial Operations in The Oil Refining Industryrogerh44No ratings yet
- UV Curing Glass Glue - Guangdong Hengda New Materials Technology-HTB1h3fHHXXXXXXOXFXX - prxfXXX4Document6 pagesUV Curing Glass Glue - Guangdong Hengda New Materials Technology-HTB1h3fHHXXXXXXOXFXX - prxfXXX4ugo_rossiNo ratings yet
- Avalanche Photodiodes PDFDocument3 pagesAvalanche Photodiodes PDFcmenikarachchiNo ratings yet
- Molecular Basis Off InheritanceDocument5 pagesMolecular Basis Off InheritanceShivam Kumar PathakNo ratings yet
- Astm B 237 - 01Document2 pagesAstm B 237 - 01kaminaljuyu100% (2)
- Effective Magnetic MomentDocument1 pageEffective Magnetic MomenttfurrowsNo ratings yet
- 012 2F PaperDocument10 pages012 2F PaperJean-Noël LerouxNo ratings yet
- AplDocument37 pagesApladitiya tegarNo ratings yet
- Lecture14 SkinEffectDocument12 pagesLecture14 SkinEffectanon_450837719No ratings yet
- Barrel Casing Type Boiler Feed Pump - SUZLONDocument41 pagesBarrel Casing Type Boiler Feed Pump - SUZLONsen_subhasis_58100% (2)
- Uncovering the Silicon - ΜL914 - Evil Mad Scientist LaboratoriesDocument21 pagesUncovering the Silicon - ΜL914 - Evil Mad Scientist LaboratoriesGulapo GapoNo ratings yet
- James B. Anderson - Quantum Chemistry by Random Walk: Higher Accuracy For H +-3Document5 pagesJames B. Anderson - Quantum Chemistry by Random Walk: Higher Accuracy For H +-3Electro_LiteNo ratings yet
- Lecture Planner - Physical Chemistry - Prayas JEE 2024Document2 pagesLecture Planner - Physical Chemistry - Prayas JEE 2024tannan75ptNo ratings yet
- Abrasive Water Jet Machining PDFDocument3 pagesAbrasive Water Jet Machining PDFbvnareshNo ratings yet
- Agilent G1316 90011 TCC A B C EbookDocument126 pagesAgilent G1316 90011 TCC A B C EbookTimor ForexNo ratings yet
- Using 222Rn To Identify and Quantify GW Discharge - Ortega Et Al - 2015Document13 pagesUsing 222Rn To Identify and Quantify GW Discharge - Ortega Et Al - 2015Noreida PRIETO MILLANNo ratings yet
- Is Water Wet - Google SearchDocument1 pageIs Water Wet - Google SearchJordyn FarmerNo ratings yet
- Quantum2 HandoutDocument188 pagesQuantum2 HandoutLizbethNo ratings yet
- Control of Flexural Cracks by Jack C. McCormacDocument9 pagesControl of Flexural Cracks by Jack C. McCormacbig_one214No ratings yet
- YaourtFoodandHealth2016 PDFDocument10 pagesYaourtFoodandHealth2016 PDFBilsiNo ratings yet
- Liquid-Liquid Equilibrium: Ternary SystemDocument29 pagesLiquid-Liquid Equilibrium: Ternary SystemaaaNo ratings yet
- Type of Business VentureDocument3 pagesType of Business VentureSasheen Dela CruzNo ratings yet
- Worksheet On Atoms, Molecules and IonsDocument7 pagesWorksheet On Atoms, Molecules and IonsTariqNo ratings yet
- Adverse Drug Reaction FormDocument2 pagesAdverse Drug Reaction FormAre Pee Etc0% (1)