Professional Documents
Culture Documents
General Organic Chemistry - DPP 01
Uploaded by
suryawanshiashish5490 ratings0% found this document useful (0 votes)
3 views3 pagesOriginal Title
General Organic Chemistry _ DPP 01
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views3 pagesGeneral Organic Chemistry - DPP 01
Uploaded by
suryawanshiashish549Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 3
1
YAKEEN-2.0
GOC
[DPP-01]
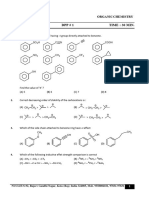
1 The inductive effect - 7. Which is the correct order of increasing basicity ?
(A) implies the atom's ability to cause bond (A) CH3CH2CH3 < CH3CH2SH < CH3CH2OH <
polarization CH3CH2NH2
(B) increases with increase of distance (B) CH3CH2CH3 < CH3CH2OH < CH3CH2SH <
(C) implies the transfer of lone pair of electrons CH3CH2NH2
from more electronegative atom to the lesser (C) CH3CH2NH2 < CH3CH2SH < CH3CH2OH <
electronegative atom in a molecule CH3CH2CH3
(D) implies the transfer of lone pair of electrons (D) CH3CH2CH3 < CH3CH2OH < CH3CH2NH2 <
from lesser electronegative atom to the more CH3CH2SH
electronegative atom in a molecule
8. The inductive effect of the groups: NH3 ; –D;
2. In which of the following compounds is hydroxylic CO3 ; –COOH are respectively
proton the most acidic ? (A) –I, +I, +I, –I
F (B) –I, –I, –I, +I
(A) O (B) I O (C) +I, No effect, –I, –I
H H
(D) +I, –I, –I, –I
O O 9. Which of the following belongs to +I group?
(C) H (D) H
F F
(A) –OMe (B) NH3
3. Decreasing – I power of given groups is -
(a) –CN (b) –NO2 (c) –NH2 (d) –F (C) (D) –OH
(A) b > a > d > c (B) b > c > d > a
(C) c > b > d > a (D) c > b > a > d 10. Choose the correct statement
(A) I effect operates in both 𝜋 and 𝜋 bonds
(B) I effect creates net charge in molecule
4. Which among the given acid has lowest pKa value - (C) I effect transfers electron from one carbon to
(A) Chloroacetic acid (B) Bromoacetic acid another
(C) Nitroacetic acid (D) Cyanoacetic acid (D) I effect creates partial charges and it is distance
dependent
5. Arrange basicity of the given compounds in
11. The increase in number of alkyl groups
decreasing order - (A) Increases the stability of carbanion
(a) CH3 – CH2 – NH2 (b) CH2 = CH – NH2 (B) Decreases the stability of carbanion
(c) CH C – NH2 (C) Remains the same
(A) a > b > c (B) a > c > b (D) None of these
(C) c > b > a (D) b > c > a 12. Arrange the following groups in the order of
decreasing (–I) effect.
6. Consider following acid (A) CN > F > Br > Cl > COOH > I > H
ClCH2COOH, CH3COOH, CH3CH2COOH (B) COOH > CN > F > Br > Cl > I > H
I II III (C) H > COOH > CN > I > Cl > F > Cl
(D) CN > COOH > F > Cl > Br > I > H
Correct order of their pH value
(A) III < II < I (B) I < II < III
(C) I < III < II (D) II < I < III
2
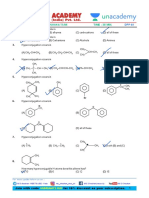
13. The +I.E. (inductive effect) is shown by: 16. The most acidic hydrogen among given compounds is:
(A) –CH3 (B) –OH
(C) –F (D) –C6H5 (A)
14. The –I effect is shown by:
(A) –COOH (B) –CH3 (B)
(C) –CH3CH2 (D) –CHR2
(C)
15. The increasing order of +ve I-effect shown by H,
CH3, C2H5 and C3H7 is:
(A) H < CH3 < C2H5 < C3H7 (D) Cannot be predicted
(B) H > CH3 > C2H5 >C3H7 17. The most acidic compound among following is:
(C) H < C2H5 < CH3 < C3H7 (A) F – CH2 – COOH
(D) None of the above (B) Cl – CH2 – COOH
(C) Br – CH2 – COOH
(D) I – CH2 – COOH
3
ANSWERS
1. (A)
2. (D)
3. (A)
4. (C)
5. (A)
6. (B)
7. (A)
8. (A)
9. (C)
10. (D)
11. (B)
12. (D)
13. (A)
14. (A)
15. (A)
16. (A)
17. (A)
Any issue with DPP, please report by clicking here- https://forms.gle/t2SzQVvQcs638c4r5
Please share your feedback on PW Teachers- https://forms.gle/jEBFswBuki4Ut2Lk6
PW Mobile APP : https://physicswala.page.link/?type=contact-us&data=open
For PW Website : https://www.physicswallah.live/contact-us
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- General Organic Chemistry(GOC) _ DPP 01_removedDocument2 pagesGeneral Organic Chemistry(GOC) _ DPP 01_removedshishiranand25No ratings yet
- Goc 6002eec9ca05fDocument14 pagesGoc 6002eec9ca05fAyush TiwariNo ratings yet
- Bakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveDocument3 pagesBakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveRitesh SonawaneNo ratings yet
- Arjuna JEE AIR (2024) GOCDocument30 pagesArjuna JEE AIR (2024) GOCON GamingNo ratings yet
- OrganicDocument9 pagesOrganicjitesh100kushwahaNo ratings yet
- Gocdpp 60Document8 pagesGocdpp 60Mrigank GuptaNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- CPP (GOC-2) RBooster ResDocument10 pagesCPP (GOC-2) RBooster RessahilNo ratings yet
- General Organic Chemistry Assignment SolutionsDocument7 pagesGeneral Organic Chemistry Assignment Solutionsrahul kmNo ratings yet
- Q.paper Aiims 2021Document190 pagesQ.paper Aiims 2021anandramNo ratings yet
- Basic Concept in Org. Chem - Exercise-Eng Module-4Document25 pagesBasic Concept in Org. Chem - Exercise-Eng Module-4Raju SinghNo ratings yet
- DPP GoccccDocument10 pagesDPP GoccccMayur Khichi0% (1)
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- PC Copy - DPP-1 (GOC)Document4 pagesPC Copy - DPP-1 (GOC)Dushyanth S JNo ratings yet
- Sheet - 02 - General Organic ChemistryDocument74 pagesSheet - 02 - General Organic ChemistrykeshavNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- Physical Properties & POC ExerciseDocument15 pagesPhysical Properties & POC ExercisergNo ratings yet
- GOC ExerciseDocument45 pagesGOC ExerciseAMAR DEEP SHUKLA100% (3)
- Organic Chemistry: Daily Practice ProblemsDocument53 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- 13DPP25FGOCEXCELMIXEDDocument5 pages13DPP25FGOCEXCELMIXEDRIP- PIRNo ratings yet
- Goc-II Ex e SiamrpnDocument22 pagesGoc-II Ex e SiamrpnArchit KhemaniNo ratings yet
- Practice Paper - Phase 3Document6 pagesPractice Paper - Phase 3Dhairya VinayakNo ratings yet
- GOC & EAS CPP-II - PMDDocument14 pagesGOC & EAS CPP-II - PMDVansh sareenNo ratings yet
- Iupac and Goc - With KeyDocument48 pagesIupac and Goc - With KeyDj chemzoneNo ratings yet
- ProjectDocument17 pagesProjectrudrashah4777No ratings yet
- Goc Sheet-3-EfDocument6 pagesGoc Sheet-3-EfAaRaV KuShWaHaNo ratings yet
- 9.GOC & IsomerismDocument37 pages9.GOC & IsomerismVinod AgrawalNo ratings yet
- UnitTest_D09-Mar-2024 (6)Document28 pagesUnitTest_D09-Mar-2024 (6)NamraNo ratings yet
- Alkyl Halide: Organic ChemistryDocument56 pagesAlkyl Halide: Organic ChemistryAaryan KeshanNo ratings yet
- Diwali Assignment 13thDocument8 pagesDiwali Assignment 13thRaju SinghNo ratings yet
- GOC document summarizes key organic chemistry conceptsDocument3 pagesGOC document summarizes key organic chemistry conceptsVanshdip RawatNo ratings yet
- Goc and Isomerism d95DOpg9PVCoDaM2Document51 pagesGoc and Isomerism d95DOpg9PVCoDaM2moviea849No ratings yet
- Guided Plan-5 (E)Document4 pagesGuided Plan-5 (E)abhiraw30062005No ratings yet
- GocDocument20 pagesGocSuyog SardaNo ratings yet
- Alkene DPPDocument20 pagesAlkene DPPKalyan ReddtNo ratings yet
- Vibrant Academy: (India) Private LimitedDocument6 pagesVibrant Academy: (India) Private LimitedRk ChaudharyNo ratings yet
- IIT-JEE HALOALKANES SN1 REACTIONS TARGETDocument44 pagesIIT-JEE HALOALKANES SN1 REACTIONS TARGETHarsh VardhanNo ratings yet
- 655f0ac18d579400185b427d_##_Aromatic Compounds Questions Notes Lakshya JEE 2.0 2024Document58 pages655f0ac18d579400185b427d_##_Aromatic Compounds Questions Notes Lakshya JEE 2.0 2024sontygaming001No ratings yet
- GOC DPPDocument35 pagesGOC DPPRayNo ratings yet
- Organic chemistry practice questionsDocument29 pagesOrganic chemistry practice questionsHet PrajapatiNo ratings yet
- DPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)Document2 pagesDPP # 09 TIME: 30 Min.: 1. Column I Column II (Compounds) (Properties)ADARSH KUMAR BEHERANo ratings yet
- Final 100 Question Bank for JEE Main 2020 ChemistryDocument29 pagesFinal 100 Question Bank for JEE Main 2020 Chemistryv k (venkat da)No ratings yet
- Alkene Exercise Eng Module-4Document21 pagesAlkene Exercise Eng Module-4Raju SinghNo ratings yet
- 14.aldehyde KetoneDocument63 pages14.aldehyde KetoneAlpha CreationNo ratings yet
- Set of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inDocument6 pagesSet of 50 Obj in General Organic Chemistry by S.K.sinha HTTP://WWW - Openchemistry.inmyiitchemistry50% (4)
- ALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyDocument4 pagesALCOHOLS, PHENOLS AND ETHERS Paper PDF Ans KeyRISHIKESH SHIRSATHNo ratings yet
- Ospp - 01Document4 pagesOspp - 01ARTH SINGHNo ratings yet
- Exercise 12Document29 pagesExercise 12Soumya AgrawalNo ratings yet
- New Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 BatchDocument23 pagesNew Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 Batchiamxxxofficial86No ratings yet
- GOC (13th)Document34 pagesGOC (13th)Raju SinghNo ratings yet
- Ms Chouhan & Team: Organic ChemistryDocument16 pagesMs Chouhan & Team: Organic ChemistryPrithviraj GhoshNo ratings yet
- Question - 123Document3 pagesQuestion - 123bazith.azasoftNo ratings yet
- PACE Final Lap (Organic Chemistry) PDFDocument152 pagesPACE Final Lap (Organic Chemistry) PDFAman AdatiaNo ratings yet
- @bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDDocument5 pages@bohring - Bot - GOC, Isomerism & EAS @HeyitsyashXDxkryxxzNo ratings yet
- Class Test-1-Aldehydes & Ketones - PreparationDocument5 pagesClass Test-1-Aldehydes & Ketones - PreparationSarthak VermaNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument7 pagesExercise-01 Check Your Grasp: O CH HO HOChesta MalhotraNo ratings yet
- Reaction IntermediatesDocument7 pagesReaction Intermediatespinnaacleclasses salemNo ratings yet
- Test Chemical BondingDocument3 pagesTest Chemical Bondingdevansh dewanNo ratings yet
- Hex A Heli Cenar Chiv Date IDocument6 pagesHex A Heli Cenar Chiv Date IKatherine PunoNo ratings yet
- Organic Chemistry Naming and StructuresDocument4 pagesOrganic Chemistry Naming and StructuresJericho CamposNo ratings yet
- Dewar BenzeneDocument7 pagesDewar BenzenechinuasfaNo ratings yet
- Tutorial Chapter 4 BioDocument9 pagesTutorial Chapter 4 BioZunnurain AmniNo ratings yet
- 2017 H2 Chemistry Paper 1 Suggested SolutionsDocument5 pages2017 H2 Chemistry Paper 1 Suggested SolutionsLee Jun HuiNo ratings yet
- Mercksindexoffin 00 MercuoftDocument192 pagesMercksindexoffin 00 Mercuoft11113432No ratings yet
- Chemistry Paper 1 FoundationDocument20 pagesChemistry Paper 1 FoundationsaadNo ratings yet
- 50 Expected QuestionsDocument6 pages50 Expected QuestionsShadhasanNo ratings yet
- Matriculation Chemistry Hydrocarbon Part 1 AlkaneDocument44 pagesMatriculation Chemistry Hydrocarbon Part 1 Alkaneiki292No ratings yet
- Alkenes NotesDocument60 pagesAlkenes NotesLenz KnightNo ratings yet
- 8 - Magnesium-GlycerophosphateDocument1 page8 - Magnesium-Glycerophosphateasmae.labindusNo ratings yet
- Alcohols, Phenols and EthersDocument4 pagesAlcohols, Phenols and EthersAnonymous GO6JVW9Wud100% (6)
- Chem 1332Document4 pagesChem 1332geoffreyrascherNo ratings yet
- 4.carbon and Its CompoundsDocument8 pages4.carbon and Its CompoundsBhai JaanNo ratings yet
- t2 Chem Revision Ex 18 Answer SchemeDocument17 pagest2 Chem Revision Ex 18 Answer SchemeNicholas OwNo ratings yet
- DPP - 06 - Substitution ReactionDocument4 pagesDPP - 06 - Substitution ReactionWhite Pubg BrothersNo ratings yet
- Stoichiometry 1Document52 pagesStoichiometry 1Mero Miro100% (1)
- Higher Order Thinking QuestionsDocument46 pagesHigher Order Thinking QuestionsAmar Minz0% (1)
- Lab Report - CHM258 - Sayyidah Nafisah BT Aiman FirdausDocument17 pagesLab Report - CHM258 - Sayyidah Nafisah BT Aiman FirdausSAYYIDAH NAFISAHNo ratings yet
- Molecular Biology - David P. ClarkDocument653 pagesMolecular Biology - David P. ClarkLaauraa13877% (30)
- Analyze Salt Acidic Basic Radicals SO42- Al3Document3 pagesAnalyze Salt Acidic Basic Radicals SO42- Al3zuhair ahmadNo ratings yet
- Pharmaceutical Organic ChemistryDocument2 pagesPharmaceutical Organic ChemistryPankaj KushwahNo ratings yet
- Experimental Dipoles - Dipole Moments in DebyeDocument82 pagesExperimental Dipoles - Dipole Moments in DebyeYourMotherNo ratings yet
- Grading Rubric For DNA ModelDocument2 pagesGrading Rubric For DNA ModelDebbie Ann LaguindabNo ratings yet
- SPM Chemistry Form 5Document5 pagesSPM Chemistry Form 5Aileen PoLyNo ratings yet
- Chem 2301 - Assignment 4 - Organic Chemistry PDFDocument20 pagesChem 2301 - Assignment 4 - Organic Chemistry PDFapi-547482481No ratings yet
- Reactions of Halides Silver Nitrate TestDocument11 pagesReactions of Halides Silver Nitrate TestLazar PopovićNo ratings yet
- Carboxylic Acids and Their DerivativesDocument22 pagesCarboxylic Acids and Their DerivativesEugene OkpanteyNo ratings yet
- McMurry OC8e EV CH15 PDFDocument18 pagesMcMurry OC8e EV CH15 PDFCrizel Ricaro100% (1)
- Solubility: Physical Pharmacy (II) - LabDocument4 pagesSolubility: Physical Pharmacy (II) - Labso soNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Chemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)From EverandChemistry: 1001 Practice Problems For Dummies (+ Free Online Practice)No ratings yet
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Transformer: The Deep Chemistry of Life and DeathFrom EverandTransformer: The Deep Chemistry of Life and DeathRating: 4.5 out of 5 stars4.5/5 (13)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)