0% found this document useful (0 votes)
289 views5 pagesFriedel Crafts Alkylation Based Ionic Liquid
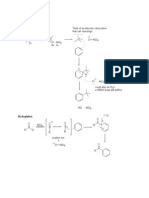
This document discusses Friedel–Crafts alkylation reactions carried out in pyridinium-based ionic liquids. The effects of various parameters like catalyst–ionic liquid composition, reactant composition, catalyst quantity, and reaction temperature were studied. The reactions proceeded under mild conditions and allowed for simple product isolation. The ionic liquids provided green characteristics to the reaction and could be recycled and reused.
Uploaded by
intata 24Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
Topics covered
- catalyst efficiency,
- reaction media,
- selectivity to products,
- product recovery,
- catalysts,
- conversion rates,
- anions,
- recycling,
- thermal stability,
- alkylation with 1-bromopropane
0% found this document useful (0 votes)
289 views5 pagesFriedel Crafts Alkylation Based Ionic Liquid
This document discusses Friedel–Crafts alkylation reactions carried out in pyridinium-based ionic liquids. The effects of various parameters like catalyst–ionic liquid composition, reactant composition, catalyst quantity, and reaction temperature were studied. The reactions proceeded under mild conditions and allowed for simple product isolation. The ionic liquids provided green characteristics to the reaction and could be recycled and reused.
Uploaded by
intata 24Copyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
Topics covered
- catalyst efficiency,
- reaction media,
- selectivity to products,
- product recovery,
- catalysts,
- conversion rates,
- anions,
- recycling,
- thermal stability,
- alkylation with 1-bromopropane