Professional Documents
Culture Documents
Solutions For Chapter6
Solutions For Chapter6
Uploaded by
kyyhnwyhc6Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solutions For Chapter6
Solutions For Chapter6
Uploaded by
kyyhnwyhc6Copyright:
Available Formats
6
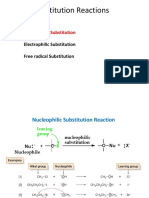
Organic Halogen Compounds; Substitution and
Elimination Reactions
Chapter Summary
Alkyl halides react with nucleophiles, reagents that can supply an electron pair to form a
covalent bond, to give a product in which the nucleophile takes the place of the halogen.
Table 6.1 gives fifteen examples of such nucleophilic substitution reactions, which can
be used to convert alkyl halides to alcohols, ethers, esters, amines, thiols, alkyl cyanides, or
acetylenes.
Nucleophilic substitution may occur by two mechanisms. The SN2 mechanism is a
one-step process. Its rate depends on the concentrations of substrate and nucleophile. If the
halogen-bearing carbon is stereogenic, substitution occurs with inversion of configuration.
The reaction is fastest for primary halides and slowest for tertiary halides.
The SN1 mechanism is a two-step process. In the first step, the alkyl halide ionizes
to a carbocation and a halide ion. In the second, fast step, the carbocation combines with
the nucleophile. The overall rate is independent of nucleophile concentration. If the halogen-
bearing carbon is stereogenic, substitution occurs with racemization. The reaction is fastest
for tertiary halides and slowest for primary halides. The two mechanisms are compared in
Table 6.2.
Elimination reactions often compete with substitution. They involve elimination of
the halogen and a hydrogen from adjacent carbons to form an alkene. Like substitution, they
occur by two main mechanisms. The E2 mechanism is a one-step process. The nucleophile
acts as a base to remove the adjacent proton. The preferred form of the transition state is
planar, with the hydrogen and the leaving group in an anti conformation. The E1
mechanism has the same first step as the SN1 mechanism. The resulting carbocation then
loses a proton from a carbon atom adjacent to the positive carbon to form the alkene.
Several polyhalogen compounds have useful properties. Among them, carbon
tetrachloride, chloroform, and methylene chloride are useful solvents. Other important
polyhalogen compounds include Halons (CBrClF2 and CBrF3) used as fire extinguishers,
and hydrochlorofluorocarbons (HCFCs), used as refrigerants, blowing agents, aerosol
propellants, and solvents. Teflon is a polymer of tetrafluoroethene. It is used in nonstick
coatings, Gore-Tex fabrics, insulators, and many other things. Certain perfluorochemicals
dissolve high percentages of oxygen and can be used as artificial blood. Many halogen-
containing compounds are important pesticides.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved. 102
Organic Halogen Compounds; Substitution and Elimination Reactions 103
Reaction Summary
Nucleophilic Substitution
Nu + R X R Nu+ + X –
–
Nu + R X R Nu + X–
(See Table 6.1 for examples)
Elimination
H
B
C C C C + BH+ + X–
base
X
Preparation of Teflon
peroxide
F2C CF2 ( CF2 CF2 )n
Mechanism Summary
SN2 (Bimolecular Nucleophilic Substitution)
+ –
Nu + C Nu C L C
+
(nucleophile) L Nu
(subtrate)
+
–
L
(leaving group)
SN1 (Unimolecular Nucleophilic Substitution)
slow fast
C + C + – L C
Nu –
L Nu
(carbocation)
or
C
Nu
E2 (Bimolecular Elimination)
B
H
BH+ + C C + – L
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
104 Chapter 6
E1 (Unimolecular Elimination)
H H
C C L C C+ C C
+ +
L – H+
Learning Objectives
1. Know the meaning of: nucleophilic substitution reaction, nucleophile, substrate,
leaving group.
2. Be familiar with the examples of nucleophilic substitution reactions listed in Table
6.1.
3. Know the meaning of: SN2 mechanism, inversion of configuration, SN1 mechanism,
racemization, rate-determining step, E2 and E1 mechanisms.
4. Know the formulas of carbon tetrachloride, chloroform, methylene chloride, Freons,
Halons, Teflon.
5. Given the name of an alkyl halide or a polyhalogen compound, write its structural
formula.
6. Given the structural formula of an alkyl halide, write a correct name for it.
7. Write the equation for the reaction of an alkyl halide with any of the nucleophiles
listed in Table 6.1. Recognize the class of organic compound to which the product
belongs.
8. Given the structure of an alkyl halide, predict whether it is most likely to react with
nucleophiles by an SN1 or an SN2 mechanism.
9. Given the structure of an alkyl halide and a nucleophile, write the equations that
illustrate the formation of both the substitution and elimination products and be able
to predict which path is likely to be favored.
10. Know the stereochemical outcome of SN1 and SN2 substitutions and E1 and E2
eliminations.
11. Given an alkyl halide with a particular stereochemistry, a nucleophile, and reaction
conditions, predict the stereochemistry of the product of nucleophilic substitution.
12. Combine nucleophilic substitutions with previously studied reactions to devise a
multi-step synthesis of a given product.
ANSWERS TO PROBLEMS
Problems Within the Chapter
_
6.1 a. NaOH + CH3CH2CH 2Br CH3CH2CH 2OH + Na+ Br
.
(item 1, Table 6.1)
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 105
_
b. (CH3CH 2)3 N + CH3CH 2Br (CH3CH 2)4 N+ Br
(item 9, Table 6.1)
c.
NaSH + CH2Br CH2 SH + Na+ Br –
(item 10, Table 6.1)
_ _
6.2 a. HO + CH 3CH2 CH2 CH2 Br CH 3CH2 CH2 CH2 OH + Br
nucleophile substrate leaving
group
(item 1, Table 6.1)
–
b. C N + ( CH3)2CHCH2Br ( CH3)2CHCH2CN + Br –
nucleophile substrate leaving
group
(item 14, Table 6.1)
+
c. (CH3 CH2CH2) 2NH + CH3 CH2 CH2Br (CH3 CH2 CH2) 3NH
_
nucleophile substrate + Br (leaving group)
(item 8, Table 6.1) This reaction is followed by the acid-base equilibrium:
+ +
(CH3 CH2CH2) 2NH + (CH3CH 2CH2 )3 NH (CH3CH2CH 2)2 NH2
+
(CH3 CH2 CH2) 3N
+ _
d. (CH3 CH2) 2S + CH3CH2Br (CH 3CH2 )3 S + Br
nucleophile substrate leaving
group
(item 12, Table 6.1)
e. I – + CH2 CHCH2Br CH2 CHCH2I + Br –
nucleophile substrate leaving
group
(item 13, Table 6.1)
f. _ _
CH3 CH2 CH2O + CH3Br CH3CH2CH 2O CH 3 + Br
nucleophile substrate leaving
group
or
_ _
CH3 O + CH3CH2CH 2Br CH3O CH2CH2CH3 + Br
nucleophile substrate leaving
group
(item 2, Table 6.1) Iodides or chlorides could also be used as substrates.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
106 Chapter 6
6.3 Use Figure 6.1 as a guide:
H
_H _
NC C Br
CH2CH 3
transition state
ENERGY
Ea
CH3CH2CH 2Br + NaCN
CH3CH 2CH 2CN + NaBr
REACTION COORDINATE
6.4 a. CH3 CH3
–
CN
CH3CH2 C C CH2CH3 + Br –
Note: inversion
H Br NC H
b. –
CN
CH3 Br H3C CN + Br –
Note: trans cis
c. CH3 CH3
Na+ – SH
H C C H + Br –
Note: inversion
CH3CH2CH2 Br HS CH2CH2CH3
6.5 CH3CH2CH2CH2Br > (CH3)2CHCH2Br > CH3CH2CH(CH3)Br
The more crowded the carbon where displacement occurs, the slower the reaction
rate.
6.6 The reaction takes place via an SN1 mechanism because the substrate is a tertiary
halide. The expected product is tert-butyl methyl ether. The reaction energy diagram
resembles that for the SN1 reaction shown in Figure 6.2:
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 107
_
(CH3)3C+Cl
+ CH3 OH
ENERGY
+
(CH3)3C–O– CH3
H
–
+ Cl
(CH3)3CCl + CH 3OH
(CH 3)3C _O_CH3 + HCl
REACTION COORDINATE
6.7 a. CH3CH2C(CH3)2Br will react faster than CH3CH2CH(CH3)Br because
ionization of the CBr bond gives the more stable carbocation (tertiary versus
secondary).
CH3 CH3 CH 3
CH3 OH
CH3CH2 C Br CH3CH 2 C + CH 3CH2 C OCH3
_
CH3 Br CH3 CH 3
+ HBr
b. Allyl bromide, CH2=CHCH2Br, will react faster than CH3CH2CH2Br because
ionization of the CBr bond gives the more stable carbocation (allylic versus
primary).
+
H2C CH CH2
CH3OH
H2C CH CH2 Br Br – H2C CH CH2 OCH3
+ + HBr
CH2CH CH2
allylic carbocation
6.8 a. SN2. The substrate is a secondary halide and may react by either SN2 or SN1.
The nucleophile HS– is a strong nucleophile, favoring SN2.
b. SN1. The substrate is a secondary halide and may react by either
mechanism. The nucleophile (CH3OH) is relatively weak and also polar,
favoring the ionization mechanism.
6.9 Two products are possible. Removal of the hydrogen from carbon-1 gives 2-methyl-
1-pentene. Removal of the hydrogen from carbon-3 gives 2-methyl-2-pentene.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
108 Chapter 6
6.10 CH3
H3CO C CH2 CH3
CH3
2-Bromo-2-methylbutane is a tertiary alkyl halide. It reacts with methanol, a weak
nucleophile and polar solvent, to give an ether by an SN1 mechanism.
ADDITIONAL PROBLEMS
6.11 a. Structures of alkyl halides can be generated by taking a hydrocarbon and
replacing one of the hydrogens with a halogen. Thus, taking propane (C3H8)
and replacing one of the hydrogens with a chlorine will generate an alkyl
halide with the molecular formula C3H7Cl. Replacement of a primary
hydrogen gives a primary halide.
CH3CH2CH2Cl 1-chloropropane
b. Start with a hydrocarbon with the molecular formula C5H12 that also has a
tertiary hydrogen.
Br
H3C C CH2 CH3
2-bromo-2-methylbutane
CH3
c. In this case we must start with a hydrocarbon with the molecular formula
C6H12. This formula indicates that the structure must also contain a double
bond or a ring. Three of the many possibilities are shown below.
I I
iodocyclohexane 3-iodo-1-hexene 4-iodo-1-hexene
6.12 Each of these reactions involves displacement of a halogen by a nucleophile. Review
Sec. 6.1 and Table 6.1.
a. CH3 CH2 CH2 CH2Br + NaI CH 3CH2 CH2 CH2I + NaBr
b. CH3 CHCH2 CH3 + NaOCH2 CH3 CH 3CHCH2CH 3 + NaCl
Cl
OCH2CH 3
c. (CH3)3 CBr + CH3 OH (CH3) 3COCH3 + HBr
The mechanism here is SN1 (most of the other reactions in this problem occur
by SN2 mechanism).
d.
Cl CH2Cl Cl CH2CN
+ +
NaCN NaCl
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 109
Substitution occurs only at the aliphatic (benzyl) carbon and not on the
aromatic ring.
e. CH3CH2CH2 I + Na+ – C CH CH3CH2CH2C CH + NaI
The use of acetylides as nucleophiles is a particularly important example of
nucleophilic substitution because it results in a new carbon–carbon bond.
Thus, larger organic molecules can be assembled from smaller ones using
this method. The same is true for cyanide ion as a nucleophile (part d).
f. CH3 CHCH3 + NaSH CH 3CHCH3 + NaCl
Cl SH
g. H2C CHCH2Cl + 2NH3 H2C CHCH2NH2 + NH4Cl
h. Br CH2CH2CH2CH2 Br +2 NaC N N CCH2CH2CH2CH2C N +2 NaBr
Displacement occurs at both possible positions.
i. CH3 CH3
+ H2O + HBr
Br OH
The starting halide is tertiary, and the mechanism is SN1.
6.13 Use the equations in Table 6.1 as a guide.
a. CH3CH2CH2Br + NH3 (item 6)
– +
b. CH3CH2I + CH3CH2S Na (item 11)
c. – + (item 15)
CH3CH2CH2Br + HC C Na
d. CH3I + CH3CH2CH2O Na+ –
(item 2)
e.
CH2Br + Na+ – C N (item 14)
f.
CH3CH2Br + O – Na+ (item 2)
6.14 The configuration inverts if the reaction occurs by an SN2 mechanism, but if the SN1
mechanism prevails, considerable racemization occurs.
a. The nucleophile is methoxide ion, CH3O–. The alkyl halide is secondary, and
the mechanism is SN2.
CH3 CH3
CH3O –
H C C H (inversion)
H3CH2C Br H3CO CH2CH3
( R ) -2-bromobutane ( S ) -2-methoxybutane
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
110 Chapter 6
b. The alkyl halide is tertiary and the nucleophile is methanol (a weaker
nucleophile than methoxide ion). The mechanism is SN1, and the product is a
mixture of R and S isomers.
CH2CH2CH3 CH2CH2CH3
– Br – CH3OH
H3C C + C
H3CH2C Br H3C CH2CH3 – H+
(S)-3-bromo-3-methylhexane
CH2CH2CH3 CH2CH2CH3
C CH3 + H3C C
H3CO CH2CH3 H3CH2C O CH3
( R ) -3-methoxy-3-methylhexane ( S ) -3-methoxy-3-methylhexane
c. The alkyl halide is secondary, and the HS– ion is a strong nucleophile. The
mechanism is SN2.
Br H
H SH _
_ + Br
H3C SH H3C
H H
(cis) (trans)
6.15 An SN2 displacement can occur. Since the leaving group and the nucleophile are
identical (iodide ion), there is no change in the gross structure of the product.
However, the configuration inverts every time a displacement occurs.
CH3 CH3
I–
H C C H + I–
H3C ( CH2)5 I H3CO ( CH2)5CH3
( R ) -2-iodobutane ( S ) -2-iodobutane
Since the enantiomer is produced, the optical rotation of the solution decreases. As
the concentration of the S enantiomer builds up, it too reacts with iodide ion to form
some R isomer. Eventually, an equilibrium (50:50) or racemic mixture is formed, and
the solution is optically inactive.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 111
6.16 The reaction involves a good nucleophile and a polar solvent (acetone).
These conditions favor an SN2 mechanism with inversion of configuration.
CH3
HS H
CH2 CH3
6.17 The first step in the hydrolysis of any one of these halides is the ionization to a t-butyl
cation:
_
(CH3 )3C X (CH3) 3C + + X
(X = Cl, Br, or I)
The product-determining step involves the partitioning of this intermediate between
two paths: one is the reaction with water and the other is loss of a proton:
H2O 뺿+
( CH3)3C OH ( CH3)3C+ H2C C( CH3)2
뺿+
Since the halide ion is, to a first approximation, not involved in these steps, this
partition occurs in the same ratio regardless of which alkyl halide is being
hydrolyzed. This result provides experimental support for the SN1 mechanism.
6.18 a. Sodium cyanide is a strong, anionic nucleophile. Thus the mechanism is SN2
and the reactivity order of the halides is primary > secondary > tertiary.
Therefore,
(CH3)2CHCH2Br > CH3CH(Br)CH2CH3 >> (CH3)3CBr
b. With 50% aqueous acetone, there is a weak nucleophile (H2O) and a highly
polar reaction medium favoring ionization, or the SN1 mechanism. In this
mechanism, the reactivity order of alkyl halides is tertiary > secondary >
primary. Therefore,
(CH3)3CBr >> CH3CH(Br)CH2CH3 >> (CH3)2CHCH2Br
6.19 a. The halide is tertiary, and the nucleophile is a relatively weak base. Hence
the predominant mechanism is SN1:
H3C Cl H3 C OCH2CH3
+ CH3CH 2OH + HCl
Some E1 reaction may occur in competition with SN1, giving mainly the
product with the double bond in the ring:
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
112 Chapter 6
H3C Cl CH 3 CH2
E1
+ CH3CH2OH + + HCl
major minor
However, the main product will be the ether (SN1).
b. The nucleophile in this case is stronger, but the SN2 process is not possible
because the alkyl halide is tertiary. This nucleophile is also a strong base.
Therefore, an E2 reaction will be preferred.
H3C Cl CH 3
H
_ E2 _
+ CH3CH2O + CH 3CH2 OH + Cl
or
Cl CH 2 H CH2
_ E2 _
+ CH3CH2O + CH 3CH2 OH + Cl
The predominant product is 1-methylcyclohexene, the more stable of the two
possible alkenes.
6.20
In the second step, the proton may be lost from either of the methyl carbons or from
the methylene carbon, giving the two alkenes shown.
6.21 The first reaction involves a strong nucleophile (CH3O–), and the SN2 mechanism is
favored. Therefore, only one product is obtained.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 113
CH3O –
O CH3
H2C CH CH CH3 H2C CH CH CH3 + Br –
Br
The second reaction involves a weak nucleophile (CH3OH) that is also a fairly polar
solvent, favoring the SN1 mechanism:
H2C CH CH CH3 H2C CH CH CH3 + Br –
+
Br
The carbocation is a resonance hybrid:
+
H2C CH CH CH3 H2C CH CH CH3
+
It can react with methanol at either positively charged carbon, giving the two
observed products.
N( CH3)2
O CH2CH3 CN
HN( CH3)2
Br
Na O CH2CH3 NaCN
KI CH3C C – Na+
I C CCH3
K + – OtBu
6.22
6.23 a. _ SN2
CH3CH2CH2O + CH3Br CH3CH2CH2OCH3 + NaBr
Na+
or
_ SN2
CH3O + CH3CH2CH2Br CH3OCH2CH2CH3 + NaBr
Na+
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
114 Chapter 6
Both reactions would be successful. This synthesis of ethers is called the
Williamson synthesis (see Sec. 8.5).
b. SN1
(CH3 )3CBr + CH3 OH (CH3 )3COCH 3 + HBr
We select this combination of reagents, not (CH3)3COH + CH3Br, because
methyl bromide, being primary, will not react by an SN1 mechanism, and
(CH3)3COH is too weak a nucleophile to displace Br– from CH3Br in an SN2
process. [(CH3)3CO–K+ would provide a strong enough nucleophile, and the
reaction
_ SN2 _
(CH3 )3CO K + + CH3 Br (CH3 )3 COCH3 + K + Br
would provide an alternative synthesis of the desired product, but it uses an
alkoxide rather than an alcohol as the problem specifies.]
6.24 Dehydrohalogenation of (bromomethyl)cyclohexane with potassium tert-butoxide, a
sterically hindered and strong base, would give the desired alkene as the major
product. The substitution derived from SN2 displacement of the halide by the alkoxide
might also be obtained as a minor product.
( CH3 )3CO – K +
CH2Br + ( CH3)3COH + KBr
Dehydrohalogenation of 1-bromo-1-methylcyclohexane with a strong base (NaOH or
potassium tert-butoxide) would also provide the desired alkene. This reaction,
however, would be complicated by the formation of methylcyclohexene as the major
product.
6.25 This type of reaction sequence is a useful method for constructing CC bonds.
a. NH3
H3C C CH + Na+NH2– H3C C C – Na+
+
SN2
CH2C C CH3 CH2Br
b. NaNH2 CH3Br
HC CH + HC C – Na+ HC C CH3
NH3
NaNH2 NH3
CH3CH2Br
CH3C C CH2CH3 CH3C C – Na+
The order in which the alkyl halides were used could be reversed, with the
same overall result.
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
Organic Halogen Compounds; Substitution and Elimination Reactions 115
6.26 a.
b. Na+ –
OH H2
H2C CHCH2Br H2C CHCH2 OH CH3CH2CH2OH
Pt
Copyright © by Brooks/Cole Cengage Learning. All rights reserved.
You might also like
- 2 Alkyl Halide SN1 & 2-HV ShahareDocument32 pages2 Alkyl Halide SN1 & 2-HV ShahareShaina 19No ratings yet
- ÔT Alkyl HalideDocument4 pagesÔT Alkyl HalideHồng HoaNo ratings yet
- SN ReactionDocument87 pagesSN ReactionSaptarshi MondalNo ratings yet
- Aliphatic Nucleophilic Substitution ReactionsDocument12 pagesAliphatic Nucleophilic Substitution ReactionsDhanaswamy Ilangeswaran100% (6)
- Alkyl HalidesDocument38 pagesAlkyl HalidesNishali SamNo ratings yet
- 2016 Lect6a Substitution Elimination (Alkilhalides)Document144 pages2016 Lect6a Substitution Elimination (Alkilhalides)Bryan SuryapranataNo ratings yet
- Organicreactionmechanism 160527094347Document55 pagesOrganicreactionmechanism 160527094347Shreyas BhandaryNo ratings yet
- Substitution Elimination (Alkilhalides) Bab 5 FessendenDocument113 pagesSubstitution Elimination (Alkilhalides) Bab 5 Fessendenahmad jamalNo ratings yet
- Group 1 - SN1Document11 pagesGroup 1 - SN1faridasaptianiNo ratings yet
- MSC Chemistry Paper-III Unit-3Document28 pagesMSC Chemistry Paper-III Unit-3DARSHANN BHESANIYANo ratings yet
- Arrow MechanismDocument49 pagesArrow MechanismtakomolyentinNo ratings yet
- Chapter 6 Nucleophilic Substitution On Saturated CarbonsDocument46 pagesChapter 6 Nucleophilic Substitution On Saturated CarbonsNUR HAZWANI BINTI MOHAMAD SANI / UPMNo ratings yet
- 100S120 CS06Document52 pages100S120 CS06b101112154No ratings yet
- UNIT 3-Organic Reactions My VersionDocument47 pagesUNIT 3-Organic Reactions My VersionMohammad JunaidNo ratings yet
- 01 1352193505 80382 PDFDocument86 pages01 1352193505 80382 PDFJennifer Carolina Rosales NoriegaNo ratings yet
- Chapter 8Document12 pagesChapter 8HarijaNo ratings yet
- Reaction Intermediates, Lectures-1 To 5Document102 pagesReaction Intermediates, Lectures-1 To 5Vasudev M SNo ratings yet
- Organic Chemistry - Chemistry of Life and Beyond ..: SynthesisDocument71 pagesOrganic Chemistry - Chemistry of Life and Beyond ..: Synthesiskrystel pyneeNo ratings yet
- Alkyl Halides PDFDocument8 pagesAlkyl Halides PDFYSOBELLATHERESE BILOLONo ratings yet
- Reagen T and Reactio N Mecha Nisms: Physic Organic ChemistryDocument34 pagesReagen T and Reactio N Mecha Nisms: Physic Organic ChemistryUun Khairunnisah KotoNo ratings yet
- Reaction Mechanisms GOC BookDocument84 pagesReaction Mechanisms GOC BookAyushNo ratings yet
- 15-Alkyl Halide-1Document7 pages15-Alkyl Halide-1ahmadmi739No ratings yet
- Chapter 7Document189 pagesChapter 7Eshita SharmaNo ratings yet
- Organic Chemistry Iit Jam PDFDocument13 pagesOrganic Chemistry Iit Jam PDFsujit patraNo ratings yet
- Chapter 6 Reactions of Haloalkanes: S 2Document8 pagesChapter 6 Reactions of Haloalkanes: S 2Roberto SIlvaNo ratings yet
- CBSE Class 12 Chem Notes Question Bank Haloalkanes and Haloarenes PDFDocument18 pagesCBSE Class 12 Chem Notes Question Bank Haloalkanes and Haloarenes PDFhehe11No ratings yet
- RX Adisi Dan SubsitusiDocument48 pagesRX Adisi Dan Subsitusistifar S1A016No ratings yet
- UltimaDocument100 pagesUltimaKrish RawatNo ratings yet
- Functional Group Transformation Using Sn2 ReactionDocument13 pagesFunctional Group Transformation Using Sn2 Reactionkurniatriwijaya.2410No ratings yet
- Nucleophilic Substitution: Nucleophilic Substitution and Elimination Reactions of Alkyl HalidesDocument99 pagesNucleophilic Substitution: Nucleophilic Substitution and Elimination Reactions of Alkyl Halidesluiji yahabaNo ratings yet
- Halogeno CompoundsDocument28 pagesHalogeno Compoundsapi-3734333No ratings yet
- Chapter Four 221212Document24 pagesChapter Four 221212Barnabas YohannesNo ratings yet
- E1 and E2 ReactionsDocument30 pagesE1 and E2 ReactionsVidhu Pandey100% (1)
- CHEM F111: General Chemistry: Semester I: AY 2017-18Document24 pagesCHEM F111: General Chemistry: Semester I: AY 2017-18shrey shahNo ratings yet
- Chemistry Model 01 SolnsDocument8 pagesChemistry Model 01 SolnsAFZ EDITZNo ratings yet
- HaloalkanesDocument37 pagesHaloalkanesYuyeen FarhanahNo ratings yet
- Synthese Et Reactivité MalonitrileDocument23 pagesSynthese Et Reactivité Malonitrilesoumeya.amrani31No ratings yet
- Nucleophilic Electrophilic Substitution Reaction in Aliphatic and Aromatic SystemsDocument9 pagesNucleophilic Electrophilic Substitution Reaction in Aliphatic and Aromatic SystemsMohammadHussainKhanNo ratings yet
- TOPIC4-Physical OrganicDocument5 pagesTOPIC4-Physical OrganicNaku MosesNo ratings yet
- Sahodaya Pre Board Examination - 2021-22: Class-XIIDocument6 pagesSahodaya Pre Board Examination - 2021-22: Class-XIIAmitNo ratings yet
- ChemistryDocument10 pagesChemistrydivyagarwal101No ratings yet
- Substitution Elemination Reactions 1Document32 pagesSubstitution Elemination Reactions 1oureducation.inNo ratings yet
- NEw WOrkDocument11 pagesNEw WOrkkaransharma690No ratings yet
- E1 and E2 Reactions 2Document23 pagesE1 and E2 Reactions 2Vidhu PandeyNo ratings yet
- Substitusi NukleofilikDocument38 pagesSubstitusi NukleofilikAde FadilahNo ratings yet
- Summary of Organic Reaction Mechanisms Needed For AS ChemistryDocument2 pagesSummary of Organic Reaction Mechanisms Needed For AS ChemistryTobeeraj PanchooNo ratings yet
- Organic Reaction MechanismsDocument47 pagesOrganic Reaction Mechanismschiomamoronu08No ratings yet
- Reaction Mechanisms: Organic ChemistryDocument76 pagesReaction Mechanisms: Organic ChemistryKhenan James NarismaNo ratings yet
- Key Words: Rearrangement Reactions, Migration ToDocument88 pagesKey Words: Rearrangement Reactions, Migration ToMonoNo ratings yet
- Classification and Nomenclature All Sheet-1Document28 pagesClassification and Nomenclature All Sheet-1sreejalakshmibinuNo ratings yet
- Chapter Four Major Organic ReactionsDocument63 pagesChapter Four Major Organic ReactionsdagmawiNo ratings yet
- Bab Benzene and Aromaticity+ (II)Document48 pagesBab Benzene and Aromaticity+ (II)ghaida farmasiNo ratings yet
- PPTDocument91 pagesPPTmelly tri rahmiNo ratings yet
- Summary of Chapter 7 HaloalkanesDocument3 pagesSummary of Chapter 7 Haloalkanesfaris zainuddinNo ratings yet
- Chapter 5 Alkyl HalidesDocument32 pagesChapter 5 Alkyl HalidesMohd HanafiahNo ratings yet
- Matriculation Chemistry (Haloalkane)Document46 pagesMatriculation Chemistry (Haloalkane)ridwanNo ratings yet
- L9 Neighbouring Group ParticipationDocument32 pagesL9 Neighbouring Group Participationvanwani.mozeelNo ratings yet
- Carbenes 170512195843Document38 pagesCarbenes 170512195843ajayyashpalNo ratings yet
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- CHM 457 Exp 2Document9 pagesCHM 457 Exp 2Ayish MataNo ratings yet
- Chapter 5 Extra Notes Part BDocument24 pagesChapter 5 Extra Notes Part BKrishnaah DorajNo ratings yet
- CHY3201 T8 Elimination ReactionsDocument16 pagesCHY3201 T8 Elimination ReactionsNURIN JAZLIENA BINTI HAZIZAN / UPMNo ratings yet
- Rev - Chemistry - AreasDocument7 pagesRev - Chemistry - AreasUppu EshwarNo ratings yet
- Alkyl Aryl Halides PDFDocument21 pagesAlkyl Aryl Halides PDFLakshit SanghrajkaNo ratings yet
- Sn12, E12 - Exam 2 Practice Problems and KeysDocument40 pagesSn12, E12 - Exam 2 Practice Problems and KeysNguyễn Khánh PhươngNo ratings yet
- Alcohols Phenols Thiols and Ethers SimpleDocument58 pagesAlcohols Phenols Thiols and Ethers Simplevin ocangNo ratings yet
- Reaction MechanismDocument20 pagesReaction MechanismPalash ChawhanNo ratings yet
- Alkenes and Cycloalkenes: Alkenes Carbon - Carbon Double BondDocument22 pagesAlkenes and Cycloalkenes: Alkenes Carbon - Carbon Double BondМария МановаNo ratings yet
- Substitution Versus Elimination Reactions: Presented By: Hina Javed Punjab Group of Colleges Rwp/IsbDocument15 pagesSubstitution Versus Elimination Reactions: Presented By: Hina Javed Punjab Group of Colleges Rwp/IsbRaja Haris JavedNo ratings yet
- RM 1 Mix QUESTION 1 230418 201559Document7 pagesRM 1 Mix QUESTION 1 230418 201559Jash ShahNo ratings yet
- PharmD 1st Year SyllabusDocument21 pagesPharmD 1st Year SyllabusAshwat ANo ratings yet
- Exp 7 Preparation of AlkenesDocument14 pagesExp 7 Preparation of AlkenesGeorge PiliposyanNo ratings yet
- (Download pdf) Conservation Of Books 1St Edition Abigail Bainbridge full chapter pdf docxDocument69 pages(Download pdf) Conservation Of Books 1St Edition Abigail Bainbridge full chapter pdf docxxafwrbekesi100% (6)
- Chapter 8 - Elimination ReactionsDocument68 pagesChapter 8 - Elimination ReactionsBianca-Rebeca PetreNo ratings yet
- Test Bank For Organic Chemistry Structure and Function 6th Edition Vollhardt DownloadDocument18 pagesTest Bank For Organic Chemistry Structure and Function 6th Edition Vollhardt Downloadchristopherwilliamsmcrjpeynzf100% (24)
- 12.2.2 Preparation of AlkenesDocument35 pages12.2.2 Preparation of AlkenesMUHAMMAD LUQMAN HAKIMI MOHD ZAMRINo ratings yet
- Chapter 5 Alkyl HalidesDocument33 pagesChapter 5 Alkyl HalidesKonoli NuingNo ratings yet
- Organic Chemistry 11Th Edition Francis A Carey Full ChapterDocument51 pagesOrganic Chemistry 11Th Edition Francis A Carey Full Chapterthomas.robinson634100% (7)
- Org Chem Lec m8 AlkylhalidesDocument5 pagesOrg Chem Lec m8 AlkylhalidesAbigail P. ARANGGANo ratings yet
- Chemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cBDocument4 pagesChemistry Organic GOC Reaction Mechanism Elimination E1 E2 E1cBRSL100% (1)
- Alkyle Halides Full ChapterDocument13 pagesAlkyle Halides Full Chapterwajid123No ratings yet
- CHEM 210 Sample Exam 3Document6 pagesCHEM 210 Sample Exam 3Varokah VarNo ratings yet
- (IIT JEE IITJEE Chemistry) Nitin D Gaikwad - Organic Reaction Mechanism Through Problem Solving Approach Nitin D Gaikwad K.T.H.M. College Nashik ISBN 978-93-5267-423-7-K.T.H.M. College Nashik (2019)Document204 pages(IIT JEE IITJEE Chemistry) Nitin D Gaikwad - Organic Reaction Mechanism Through Problem Solving Approach Nitin D Gaikwad K.T.H.M. College Nashik ISBN 978-93-5267-423-7-K.T.H.M. College Nashik (2019)Nia100% (1)
- Pharmaceutical Organic Chemistry1Document4 pagesPharmaceutical Organic Chemistry1vinay0717No ratings yet
- Alkyl Halide (Quiz Cum Practice Test)Document2 pagesAlkyl Halide (Quiz Cum Practice Test)Akshit SharmaNo ratings yet
- Chapter 1 Amine in ClassDocument72 pagesChapter 1 Amine in ClassChiu FongNo ratings yet
- Alkil Halida Ko - 1 BaruDocument101 pagesAlkil Halida Ko - 1 BaruWahyudi PrasetiantoNo ratings yet
- SN1 SN2 E1 E2 Reaction PHR-122Document36 pagesSN1 SN2 E1 E2 Reaction PHR-122zakariansu67% (6)
- Organic 2 Organic Chemistry Wade 69 CH 7 Structure and Synthesis of Alkenes 2622Document9 pagesOrganic 2 Organic Chemistry Wade 69 CH 7 Structure and Synthesis of Alkenes 2622Gagandeep SinghNo ratings yet