100% found this document useful (4 votes)
4K views2 pagesDisplacement Reactions Worksheet
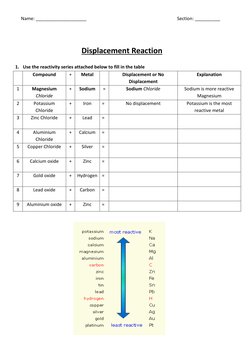
The document discusses displacement reactions using a reactivity series table. Students are asked to fill in the table indicating whether displacement will occur. They are also asked questions about a copper wire reacting in silver sulfate solution and extracting metals from ores using carbon.
Uploaded by
ABDULRAHMAN MOUSLLICopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
100% found this document useful (4 votes)
4K views2 pagesDisplacement Reactions Worksheet
The document discusses displacement reactions using a reactivity series table. Students are asked to fill in the table indicating whether displacement will occur. They are also asked questions about a copper wire reacting in silver sulfate solution and extracting metals from ores using carbon.
Uploaded by
ABDULRAHMAN MOUSLLICopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Displacement Reaction: Provides a table to document displacement reactions based on a reactivity series, highlighting the most and least reactive elements.
- Chemical Reaction Questions: Presents theoretical questions to explore concepts of reactivity and reaction types using examples of elements like copper and carbon.