Professional Documents
Culture Documents
235 Practice Exam 1
Uploaded by
bamforOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
235 Practice Exam 1
Uploaded by
bamforCopyright:
Available Formats
Practice Questions for 32-235 Exam 1 Note these are for reviews only.
Make sure that you can do all homework without consulting the Solutions Manual and that you can complete them in a reasonable amount of time (speed of essence). Multiple Choices (Choose only ONE answer !) 1. What kind of molecular orbital results when the two atomic orbitals shown below interact in the manner indicated?
(a) * 2.
(b)
(c) *
(d)
(e) none of the above
Which of the following matches of names and molecules are correct ? A. Alcohol B. Aldehyde C. Ketone D. Acid (a) C and III, D and IV (c) A and III, B and IV (e) none of the above I. HCOOH II. (CH3)3COH III. CH3OCH3 IV. CH3COCH3 (b) A and II, C and IV (d) B and II, D and I
3.
The number of bonds in the molecule shown below is CH3CH=CHCH2C CH (a) 1 (b) 2 (c) 3 (d) 4 (e) 5
4.
The relationship of the following two structures is H3C Br (a) structural isomers (d) not isomeric Br C C CH3 H3C Br C C CH3 Br
(b) geometric isomers (c) the same (e) conformational isomers
5.
Which are the correct orbital hybridizations for the carbon atoms in the following structures ? CH3CH3 sp3 I (a) I, II, III (e) I, III, V CH2=CH2 sp2 II (b) I, II, IV CH3 sp2 III (c) II, III, V
+
HC N sp2 IV
H2O sp V (d) III, IV, V
6.
Which of the following compounds is nonpolar (dipole moment = 0) ? Cl H2O (a) NH3 (b) CH3Cl (c) C H (d) C H Cl
7.
(e) none of the above Which compound would have the lowest boiling point ? OH OH (a) CH2OH OH (e) Consider the three isomeric alkanes n-hexane, 2,3-dimethylbutane, and 2methylpentane. Which of the following correctly lists these compounds in order of increasing boiling point? a) b) c) d) d) 2,3-dimethylbutane < 2-methylpentane < n-hexane 2-methylpentane < n-hexane < 2,3-dimethylbutane 2-methylpentane < 2,3-dimethylbutane < n-hexane n-hexane < 2-methylpentane < 2,3-dimethylbutane n-hexane < 2,3-dimethylbutane < 2-methylpentane (d)
O (b) (c)
8.
Page 2 of 11
9.
How many tertiary hydrogens are there in the following structure:
(a) 1 10.
(b) 2
(c) 3
(d) 4
(e) 5
Which of the following pairs of formula represent structural isomers ? F (a) and F
F (b) F H
HH F and
F F H
F HH
(c) d) e) 11.
CH3CH2CH2NH2
and
(CH3)3N
More than one of these None of these
Which of the following is a representation of the gauche conformer of butane. CH3 CH3 H3C CH3 H H H3C H (d) H H CH H 3
H H
H H
H3C H
H H
CH3 (a)
H (b)
H H (c)
(e) none of the above 12. Which of the above conformations is the least stable ? (a) (b) (c) (d) (e)
Page 3 of 11
13.
Which drawing represents the most stable conformation of cis-1-tert-butyl-4methylcyclohexane. CH3 C(CH3)3 C(CH3)3 CH3 C(CH3)3 CH3 C(CH3)3 (c) (d)
CH3
(a) (e) none of the above
(b)
14.
If stronger bonds are broken and weaker bonds are formed, then the reaction is (a) is exothermic (d) has a negative S (b) is endothermic (e) is spontaneous (c) has a positive S
15.
Which of the following molecules can exist as geometric isomers? CH3 (A) CH3 (D) CH3CH2CHCH3 Cl (B) CH2 C Cl H (C) CHCH2CH3
(E) CH3CH CHCH3
16.
The relationship between the following two structures is H H H H C CH2 C H CH3CH2 C H CH2CH3 CH3
CH3 H H
CH2 C
(A) same (B) constitutional isomers (C) geometric isomers (D) not isomers (E) structural isomers
Page 4 of 11
17.
Which is an acceptable resonance structure for the following drawing? H C O H CH3 (A) CH3CH OH (D) CH3CH OH (B) CH2 O CH3 (C) CH3CH2O
(E) None of the above
18.
The relationship of the following two structures is H3C H (A) structural isomers (D) not isomeric CH3 H H H3C CH3 H (C) the same
(B) conformational isomers (E) stereoisomers
19.
Which is(are) the correct orbital hybridization(s) for the C and N atoms in the following structures ? CH3CH3 sp3 I (A) I (B) II, III CH2=CH2 sp II (C) III, IV NH3 sp2 III (D) I, III HC CH
sp2 IV (E) II
20.
The b.p. of the following compounds is expected to decrease in this order (highest b.p. first): CH3CH2CH2CH3 I CH3CH2CHCH3 CH3 III (A) II > III > IV > I (D) IV > II > III > I (B) III > I > II > IV (E) I > II > III > IV IV (C) III > II > IV > I CH3CH2CH2CH2CH3 II CH3CH2CH2CH2OH
Page 5 of 11
21.
An acceptable name for the following structure is
(A) 8-t-Butyl-4-ethyl-3-methyldecane (C) 3,7-Diethyl-2,2,8-trimethyldecane (E) none of the above 22.
(B) 3-sec-Butyl-7-tert-butylnonane (D) 4,8-Diethyl-3,9,9-trimethyldecane
Which statement is true regarding the following conformations for butane? H H CH3 CH3 (A) (B) (C) (D) (E) H H H3CCH3 H H H H H H CH3 III CH3 H H
I II I is less stable than II because of steric strain III is less stable than I due to its torsional strain III is called gauche conformation The steric strain in II is greater than in I III has more energy than II
Page 6 of 11
23.
Which of the following molecules is the least stable ? CH3 H CH3 (A) H CH3 (D) H CH3 H3C H (E) H H CH3 H3C H (B) CH3 H CH3 H (C) H CH3
24.
Which name(s) is(are) acceptable for the following compound? Cl CH3
(A) (B) (C) (D) (E) 25.
5-chloro-2-methylcyclohexane 4-chloro-2-methylcyclohexane 1-chloro-3-methylcyclohexane 2-chloro-5-methylcyclohexane More than one of these
Which of the following represents the most stable conformation of cis-1isopropyl-4-methylcyclohexane? CH(CH3)2 H CH3 (A) H H CH3 (C) CH(CH3)2 H3C H (D) H H3C H (B) CH(CH3)2 H H CH(CH3)2
(E) None of these fits the name given Page 7 of 11
26.
How many tertiary hydrogen(s) are(is) there in the following structure?
(A) 1 27.
(B) 2
(C) 3
(D) 6
(E) 10
The strength of the following bases decreases in the order of (strongest first): O CH3O I (A) I > IV > III > II (D) IV > I > II > III CH3 NH2 CH3C O IV (C) II > I > III > IV
II III (B) III > IV > I > II (E) II > III > I > IV
28.
Which molecule is expected to have the largest molecular dipole moment ? H Cl BH3 CH3Cl Cl H (C) (B) (A) CCl4 (D) F C C F
(E)
29.
The formal charge on nitrogen in the Lewis structure below is H H H H C H (A) +2 (B) +1 (C) 0 C N H (D) -1 (E) -2
30.
Considering the following acid-base reaction, which statement is NOT true? CH3 O C O H + NH3 CH3 O C O + NH4
+
31.
IV III I II pKa=9.2 pKa = 4.7 (A) I is more acidic than IV (B) II is more basic than III (C) The equilibrium favors the right (D) The two C-O bonds in III are of different length. (E) more than one of the above When the 1s orbitals of two hydrogen atoms combine to form a hydrogen Page 8 of 11
molecule, how many molecular orbitals are formed ? (A) one 32. (B) two (C) three (D) four (E) five
The nitrogen atom in the molecule below is sp2 hybridized. What is the geometry of the atoms connected to nitrogen ? CH2 (A) square (D) linear (B) tetrahedral (E) pyramidal NH2 (C) trigonal planar
33.
Which of the following is the best description for the C=N bond above? (A) It results from sp2(C)-sp2(N) overlap (B) It results from sp3(C)-sp2(N) overlap (C) The two bonds are of same property (D) One bond is from sp2(C)-sp2(N) and the other from 2 p orbitals (E) None of the above
34.
The relationship of the following two molecules is best described by CH3 CH3
(A) structural isomers (C) not isomers (E) conformational isomers 35.
(B) geometric isomers (D) homologous
Which of the following bonds can act to form hydrogen bonding (A) O-H (B) N-H (E) more than one the above (C) C-H (D) C-C
36.
The boiling point of straight chain alkanes increases as the number of carbons increases. This is because (A) the larger the molecule, the greater the dipole moment (B) Larger molecules usually can form hydrogen bonding better (C) Larger molecules can pack tightly together (D) Larger molecules have greater surface therefore stronger London Forces among them. (E) None of the above
Page 9 of 11
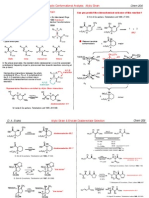
Essay Questions 1. Use the curved arrow formalism(electron pushing) to indicate the movement of electron pairs in the following reaction. Indicate in the boxes which reactants are nucleophile (Nu) and/or electrophile (E). CH3CH2O + CH3 I CH3CH2OCH3 + I
2.
Draw the important resonance contributing forms for the structure shown below. (Use electron arrow pushing for help) O
3.
Give an acceptable IUPAC name for the following compound:
4. (A) Write the two chair conformations for cis-1-tert-butyl-2-ethylcyclohexane (the following two templates are drawn to help you)
(B) In the above conformations, label the positions occupied by tert-butyl and ethyl groups by using "ax" for axial and "eq" for equatorial positions. (C) Determine which conformation would have a higher energy and briefly explain the reason. Page 10 of 11
5. The three compounds below have identical or nearly identical molecular mass. Rank them in the increasing order (lowest first) of their boiling points, and briefly explain your reasoning. CH3CH2CH2CH2OH CH3CH2OCH2CH3 HOCH2CH2OH
A B C 6. Draw the Lewis structures for three compounds with a formula of C3H8O. What is the relationship among these three compounds?
7. Redraw each molecule below to show lone pair electrons, then arrange them in the order of increasing basicity (lowest first, use <). Explain your reasoning for the order. O CH3CH2O A CH3CH2NH B CH3C O C
8. Draw the important resonance structures for the following molecule. (hint: try electron arrow pushing) O H C CH CH CH2
Page 11 of 11
You might also like
- Stereochemistry QustionsDocument43 pagesStereochemistry QustionsSwaraj Paul100% (1)
- Mechanism in Advance Organic ChemistryDocument334 pagesMechanism in Advance Organic Chemistrye.mottaghi100% (4)
- CH13 Hydrocarbons Shobhit NirwanDocument58 pagesCH13 Hydrocarbons Shobhit NirwanpujaNo ratings yet
- babb813b-8c03-4c8c-ba0a-dc9d522c9a32Document69 pagesbabb813b-8c03-4c8c-ba0a-dc9d522c9a32Navkar ShahNo ratings yet
- Organic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The SDocument9 pagesOrganic Chemistry 32-235 Practice Questions For Exam #2: 2. Consider The Ssweta KushwahaNo ratings yet
- Moon - Exam 2 - Summer 2011Document10 pagesMoon - Exam 2 - Summer 2011Andres Pena100% (2)
- Organic Chemistry Assignment QuestionsDocument21 pagesOrganic Chemistry Assignment QuestionsChocolaMeilleurNo ratings yet
- Chemistry 261 Quiz 3 Practice Fall 2013Document14 pagesChemistry 261 Quiz 3 Practice Fall 2013Natasha Moo100% (2)
- CHEM 281 Practice Exam TitleDocument12 pagesCHEM 281 Practice Exam TitleRam KrishnaNo ratings yet
- of HydrocarbonsDocument45 pagesof HydrocarbonsSneha KediaNo ratings yet
- Exam Organic Chemistry I WhittenDocument19 pagesExam Organic Chemistry I WhittenDaniel Baylosis Asong60% (5)
- Fall 2008 Old - Exam - 4Document12 pagesFall 2008 Old - Exam - 4alfredNo ratings yet
- MCQs pdf-1 PDFDocument5 pagesMCQs pdf-1 PDFEmman Ann100% (3)
- Organic Chemistry I Exam 4 20101 KeyDocument15 pagesOrganic Chemistry I Exam 4 20101 KeyAlicia ShortNo ratings yet
- The Chemistry of Fullerenes PDFDocument285 pagesThe Chemistry of Fullerenes PDFsadfsdafNo ratings yet
- Organic Chemistry 231 Final ExamDocument19 pagesOrganic Chemistry 231 Final ExamAlex Rose100% (1)
- 235practice Exam 1Document11 pages235practice Exam 1Zia RathoreNo ratings yet
- 15 CHEMISTRY Some Basic Principles & Techniques HydrocarbonsDocument3 pages15 CHEMISTRY Some Basic Principles & Techniques HydrocarbonsHasan shaikhNo ratings yet
- Solomons Testbank1 Struktur Bindn + SvarDocument7 pagesSolomons Testbank1 Struktur Bindn + SvarTahirat NasiruNo ratings yet
- Organic Chemistry I Practice Exam ADocument13 pagesOrganic Chemistry I Practice Exam ANoleNo ratings yet
- ORGANIC20CHEMISTRY20POST20TESTDocument13 pagesORGANIC20CHEMISTRY20POST20TESTJan Mill100% (1)
- Organic Chemistry: Basic Principles & TechniquesDocument4 pagesOrganic Chemistry: Basic Principles & TechniquesHasan shaikhNo ratings yet
- MCDocument33 pagesMCRachel AnneNo ratings yet
- Test Bank For Organic Chemistry 8th Edition by CareyDocument15 pagesTest Bank For Organic Chemistry 8th Edition by CareyChristine Rivera100% (30)
- AP Chemistry: Bonding Multiple ChoiceDocument5 pagesAP Chemistry: Bonding Multiple ChoiceSyed Abdul Rehman ShahNo ratings yet
- Different Sample Multiple Choice Questions in General Chemistry and Organic ChemistryDocument9 pagesDifferent Sample Multiple Choice Questions in General Chemistry and Organic ChemistryGeorge Isaac McQuilesNo ratings yet
- CH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesDocument8 pagesCH 331/1 Midterm Examination/Summer 2013/Dr. Daniel J. T. MylesAnita MarLaNo ratings yet
- Prof Shekhar ChemistryDocument9 pagesProf Shekhar Chemistryveer_sNo ratings yet
- IsomerismDocument16 pagesIsomerismAnusmita MukherjeeNo ratings yet
- Test Bank For Organic Chemistry 8th Edition by CareyDocument16 pagesTest Bank For Organic Chemistry 8th Edition by Careysinopervertext08iNo ratings yet
- CHM 1321 Assignment 1 Answers: CN H H H H HDocument10 pagesCHM 1321 Assignment 1 Answers: CN H H H H HSara YuenNo ratings yet
- C) 2-Methyl-2-Propanol and Isobutyl AlcoholDocument4 pagesC) 2-Methyl-2-Propanol and Isobutyl AlcoholZia RathoreNo ratings yet
- Problems - SET-1 Org Without AnswersDocument19 pagesProblems - SET-1 Org Without AnswersNidhi SisodiaNo ratings yet
- Section A: Multiple Choice Questions: 1s 2s 2p 2p 2p 1s 2s 2p 2p 2pDocument9 pagesSection A: Multiple Choice Questions: 1s 2s 2p 2p 2p 1s 2s 2p 2p 2pGemsNo ratings yet
- Organic Chemistry Final Exam ReviewDocument13 pagesOrganic Chemistry Final Exam ReviewHamed AliNo ratings yet
- WS Class 11 Org ChemDocument4 pagesWS Class 11 Org ChemJavedNo ratings yet
- Organic Problems1Document9 pagesOrganic Problems1Sung-Eun KimNo ratings yet
- BondingDocument7 pagesBondingtinsae workuNo ratings yet
- Questions Chapter 1-10Document107 pagesQuestions Chapter 1-10PriyaranjanNo ratings yet
- Quiz 5 Answer KeyDocument6 pagesQuiz 5 Answer KeycwodNo ratings yet
- IsomerismDocument21 pagesIsomerismkaransharma690No ratings yet
- Exercise 2Document23 pagesExercise 2Tushar RajNo ratings yet
- Electronic Structure and Covalent Bonding: Essentials of Organic Chemistry (Bruice)Document33 pagesElectronic Structure and Covalent Bonding: Essentials of Organic Chemistry (Bruice)tyron9520No ratings yet
- CH102 Principles and Reactions in Organic Chemistry: Fste School of Biological and Chemical SciencesDocument13 pagesCH102 Principles and Reactions in Organic Chemistry: Fste School of Biological and Chemical SciencesTetzNo ratings yet
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- Ann QP 11Document4 pagesAnn QP 11technical SiteNo ratings yet
- Bhayankar SawaalDocument6 pagesBhayankar SawaalNidhi SisodiaNo ratings yet
- Term1 Class Xi QN Paper 2021Document11 pagesTerm1 Class Xi QN Paper 2021Raj IgniteZ SisoudiaNo ratings yet
- Chap 1 AssignDocument7 pagesChap 1 AssignJianqi NiHao ChenNo ratings yet
- 94 433Document5 pages94 433khalidibrahimNo ratings yet
- Lewis Structures and Formal ChargesDocument18 pagesLewis Structures and Formal ChargesMarcos ViníciusNo ratings yet
- Acidicity Basicity & H - Bonding Tautomerism (Q.B.) 13thDocument16 pagesAcidicity Basicity & H - Bonding Tautomerism (Q.B.) 13thRaju SinghNo ratings yet
- Block Test-I Chemistry Class Xi 2021-22Document10 pagesBlock Test-I Chemistry Class Xi 2021-22Soham NagNo ratings yet
- Class Test - Structural IsomersDocument3 pagesClass Test - Structural IsomersAlex SamNo ratings yet
- TestbankDocument6 pagesTestbankRen H. RoxasNo ratings yet
- XII CHEMISTRY Pre Board 2 - 2023Document6 pagesXII CHEMISTRY Pre Board 2 - 2023VOLTZNo ratings yet
- Multiple-choice Questions on Organic Chemistry ConceptsDocument37 pagesMultiple-choice Questions on Organic Chemistry ConceptsTheo CaldasNo ratings yet
- Chemistry Mock ExamDocument6 pagesChemistry Mock ExamLauraNo ratings yet
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Sample Paper Gr11Document3 pagesSample Paper Gr11Enoca AJNo ratings yet
- Conformational Analysis of Organic CompoundsDocument42 pagesConformational Analysis of Organic CompoundsHASRHONNo ratings yet
- Stereoisomerization and Homolytic Decomposition of Cis and Trans Bridgehead Diazenes1 2Document10 pagesStereoisomerization and Homolytic Decomposition of Cis and Trans Bridgehead Diazenes1 2Nabil KhalidNo ratings yet
- 2m Engl Isomerism 2021 For StudDocument96 pages2m Engl Isomerism 2021 For StudGhost ShooterNo ratings yet
- DimethylpananthereneDocument11 pagesDimethylpananthereneMD Redwan IslamNo ratings yet
- Chemistry Code SS 41Document12 pagesChemistry Code SS 41Abhinav AgarwalNo ratings yet
- Chapter 4Document33 pagesChapter 4채종희No ratings yet
- Chelate Ring Size and Metal Ion SelectionDocument7 pagesChelate Ring Size and Metal Ion SelectionGuillermoNo ratings yet
- (David Morris) Stereochemistry Tutorial Chemistry PDFDocument182 pages(David Morris) Stereochemistry Tutorial Chemistry PDFAditya PrakashNo ratings yet
- Unit III: Alkanes and Cycloalkanes: E. D. Gloria Ust - Faculty of Pharmacy Chem200 - Organic ChemistryDocument19 pagesUnit III: Alkanes and Cycloalkanes: E. D. Gloria Ust - Faculty of Pharmacy Chem200 - Organic ChemistryLyra LasangreNo ratings yet
- Curso de Quimica IDocument233 pagesCurso de Quimica IMatt Durán CarusoNo ratings yet
- Chem Part 1Document83 pagesChem Part 1Rhea Monette LosentesNo ratings yet
- Topics in Current Chemistry: Managing Editor: F. L. BoschkeDocument212 pagesTopics in Current Chemistry: Managing Editor: F. L. Boschkemadbois 420No ratings yet
- (Structure and Bonding Bioinorganic Chemistry - (1990)Document232 pages(Structure and Bonding Bioinorganic Chemistry - (1990)Mary LorenaNo ratings yet
- Allylic StrainDocument18 pagesAllylic StrainRahn NaNo ratings yet
- Chapter 3 McmurryDocument26 pagesChapter 3 Mcmurrymuhammad_asim_10No ratings yet
- 11.konformasi Alkana Dan SikloalkanaDocument25 pages11.konformasi Alkana Dan SikloalkanasatriaramdhaniNo ratings yet
- McMurry9e PPT CH03Document48 pagesMcMurry9e PPT CH03김가영No ratings yet
- IsomerismDocument61 pagesIsomerismSaket DubeyNo ratings yet
- Chapter 4 Study Guide PDFDocument57 pagesChapter 4 Study Guide PDFkNo ratings yet
- Baeyrs Strain TheoryDocument5 pagesBaeyrs Strain TheorySk ZNo ratings yet
- SRG Major Test Paper 12-12-2023Document28 pagesSRG Major Test Paper 12-12-2023pal018488No ratings yet
- D.nasipuri Chapter 1Document14 pagesD.nasipuri Chapter 1Sandipan Saha100% (1)
- Introduction To Heterocyclic Chemistry: 1.1 CoverageDocument17 pagesIntroduction To Heterocyclic Chemistry: 1.1 CoverageFlavio SantosNo ratings yet
- Conformations of Alkanes and CycloalkanesDocument18 pagesConformations of Alkanes and CycloalkanesjuanNo ratings yet
- Cyclic AlkanesDocument35 pagesCyclic AlkanesKunjal100% (2)