0% found this document useful (0 votes)
19 views3 pagesCarnot Cycle Explained: T-S Diagram Insights
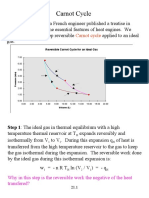
The document discusses the temperature-entropy (T-S) diagram used to illustrate thermodynamic cycles, particularly the Carnot cycle. It explains the relationship between heat transfer, work done, and efficiency in thermodynamic processes, highlighting how these are represented graphically. The efficiency of a Carnot cycle is defined in relation to the heat exchanged and the temperatures of the reservoirs involved.
Uploaded by
Irshad AhmadCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
19 views3 pagesCarnot Cycle Explained: T-S Diagram Insights
The document discusses the temperature-entropy (T-S) diagram used to illustrate thermodynamic cycles, particularly the Carnot cycle. It explains the relationship between heat transfer, work done, and efficiency in thermodynamic processes, highlighting how these are represented graphically. The efficiency of a Carnot cycle is defined in relation to the heat exchanged and the temperatures of the reservoirs involved.
Uploaded by
Irshad AhmadCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd