Experiment No:
Date:
DEPARTMENT OF CHEMISTRY
GITAM School of Science, Visakhapatnam campus
Name: P. Kameshwari
Branch:B.Sc.Chemistry
Registration Number:122012806012 Duration: 90 min.
DETERMINATION OF CELL CONSTANT
Aim: To determine the cell constant for a given cell at room temperature.
Apparatus: Conductivity bridge, conductivity cell, burette, pipette, volumetric flasks and
100mL beaker.
Chemicals: KCl
Principle:
Electrolyte solutions obey Ohm’s law i.e., the current (I) passing through a solution is
proportional to the applied potential difference.
E
I=
R
Where, E is the potential difference in volts, and R is the resistance measured in ohms (or Ω).
The resistance R of a conductor is directly proportional to its length, l, and inversely
proportional to the area of its cross-section, A.
l l A
R∝ or R=ρ ⇒ ρ=R
A A l
Where, ρ “rho” is a constant of proportionality and is called resistivity or specific resistance.
The reciprocal of the resistance is called conductance. It is denoted by C.
1
C=
R
Unit of conductance is ohm-1 or mho or Siemen(S)
The reciprocal of specific resistance is termed Specific conductance or Specific conductivity.
1 1 l l
κ= = X =C
ρ R A A
The conductance of one centimetre cube (cc) of a solution of an electrolyte.
Units of Specific conductance ohm–1cm–1 or S cm–1
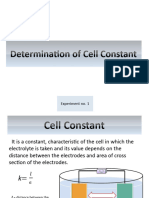
Cell constant for a cell is defined as the constant factor which stands for the ratio of the
specific conductance of a solution and its measured conductance in the cell.
l κ
Cell constant = =
A C
The cell constant is determined by measuring the conductance of a cell filled with a solution
of known specific conductance, which is invariable KCl.
�Experiment No:
Date:
DEPARTMENT OF CHEMISTRY
GITAM School of Science, Visakhapatnam campus
Procedure
Prepare 1M KCl solution by weighing accurately 7.455 gm of KCl into a clean 100 ml
standard flask and add deionised water to make up the volume upto the mark. From this 1M
KCl solution prepare 100ml each of 0.1 M, 0.02M, 0.01M, using the dilution method as
following.
0.1 M KCl: Take 10 ml of 1 M KCl into a clean 100 ml standard flask and add deionised
water to make up the volume till the mark to get 100 ml solution.
0.02 M KCl: Take 20 ml of 0.1 M KCl into a clean 100 ml standard flask and add deionised
water to make up the volume till the mark to get 100 ml solution.
0.01 M KCl: Take 10 ml of 0.1 M KCl into a clean 100 ml standard flask and add deionised
water to make up the volume till the mark to get 100 ml solution.
Do the necessary connections in the conductivity meter and wash the electrode with deionised
water. Wipe the electrode with a tissue paper. Take about 40ml of each solution in to a clean
and dry 100ml beaker and dip the conductivity cell. Measure the conductance of each solution
and note down. Note the specific conductance values of each of the solution from literature.
Then calculate the cell constant as average of all value and from the slope of the graph
Specific conductance vs observed conductance.
Table:
Sl. No Concentration Specific Conductance Observed Cell Constant
(M) (S cm–1) Conductance (S) (cm–1)
1 1 99.5 1.1236
111.80
2 0.1 12.76 1.0094
12.88
3 0.02 2.765 2.676 1.0332
4 0.01 1.414 1.357 1.0420
Average Cell constant 1.052
Result :
The cell constant of the given conductivity cell obtained from the average = …1.052……….
cm–1 and from the slope of the graph = …1.125………. cm–1
Evaluation of result
Sample Experimental value Actual Value Percentage of error Marks awarded
number
�Experiment No:
Date:
DEPARTMENT OF CHEMISTRY
GITAM School of Science, Visakhapatnam campus