The Rate-determining Step
Most reactions take place in more than one step. These separate steps are known as the
reaction mechanism
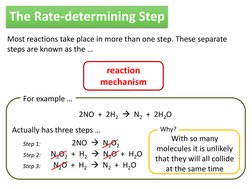
For example
2NO + 2H2 N2 + 2H2O Actually has three steps
Step 1:
Step 2: Step 3:
Why?
2NO N2O2 N2O2 + H2 N2O + H2O N2O + H2 N2 + H2O
With so many molecules it is unlikely that they will all collide at the same time
�The Rate-determining Step
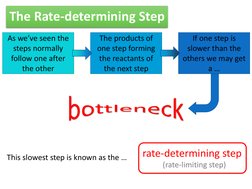
As weve seen the steps normally follow one after the other The products of one step forming the reactants of the next step If one step is slower than the others we may get a
This slowest step is known as the
rate-determining step
(rate-limiting step)
�The Rate-determining Step
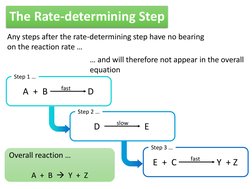
Any steps after the rate-determining step have no bearing on the reaction rate and will therefore not appear in the overall equation
fast
Step 1
A + B
D
Step 2 slow
D
Overall reaction
E
Step 3 fast
E + C
Y +Z
A + B Y + Z
�The Rate-determining Step
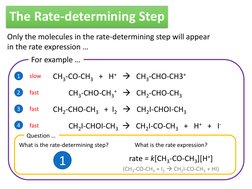
Only the molecules in the rate-determining step will appear in the rate expression For example
1 2 3 4 slow fast fast
CH3-CO-CH3 + H+ CH3-CHO-CH3+ CH3-CHO-CH3+ CH2-CHO-CH3 CH2-CHO-CH3 + I2 CH2I-CHOI-CH3 CH2I-CHOI-CH3 CH2I-CO-CH3 + H+ + IWhat is the rate expression?
fast
Question What is the rate-determining step?
rate = k[CH3-CO-CH3][H+]
(CH3-CO-CH3 + I2 CH2I-CO-CH3 + HI)
�The Rate-determining Step
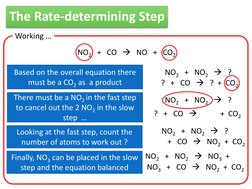
What can we work out if we are told the following 2 step reaction NO2 + CO NO + CO2 rate = k[NO2]2 Working rate = k[NO2]2 = [NO2] x [NO2]
Since this is the only species in the rate equation the slow step must be The fast step must look something like this
NO2 + NO2 ?
? + CO ?
�The Rate-determining Step
Working NO2 + CO NO + CO2 Based on the overall equation there must be a CO2 as a product There must be a NO2 in the fast step to cancel out the 2 NO2 in the slow step Looking at the fast step, count the number of atoms to work out ? NO2 + NO2 ? ? + CO ? + CO2 NO2 + NO2 ? ? + CO NO2 + CO2 NO2 + NO2 ? NO3 + CO NO2 + CO2
Finally, NO3 can be placed in the slow NO2 + NO2 NO3 + NO NO3 + CO NO2 + CO2 step and the equation balanced
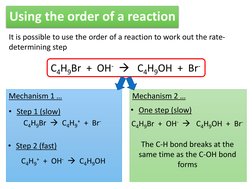
�Using the order of a reaction
It is possible to use the order of a reaction to work out the ratedetermining step
C4H9Br + OH- C4H9OH + BrMechanism 1 Step 1 (slow) C4H9Br C4H9+ + Br Step 2 (fast) C4H9+ + OH- C4H9OH Mechanism 2 One step (slow) C4H9Br + OH- C4H9OH + Br-
The C-H bond breaks at the same time as the C-OH bond forms
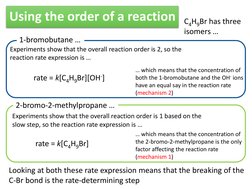
�Using the order of a reaction
1-bromobutane
Experiments show that the overall reaction order is 2, so the reaction rate expression is
C4H9Br has three isomers
rate = k[C4H9Br][OH-]
which means that the concentration of both the 1-bromobutane and the OH- ions have an equal say in the reaction rate (mechanism 2)
2-bromo-2-methylpropane
Experiments show that the overall reaction order is 1 based on the slow step, so the reaction rate expression is
rate = k[C4H9Br]
which means that the concentration of the 2-bromo-2-methylpropane is the only factor affecting the reaction rate (mechanism 1)
Looking at both these rate expression means that the breaking of the C-Br bond is the rate-determining step
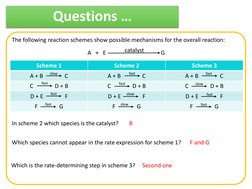
�Questions
The following reaction schemes show possible mechanisms for the overall reaction: A + E Scheme 1 A+B C F
fast slow
catalyst Scheme 2
G Scheme 3
fast
C F G
A+B C F
fast
fast
C F G
A+B C F
slow
C F G
D+B
fast
D+B
slow
D+B
fast
D+E
D+E
D+E
fast
slow
fast
In scheme 2 which species is the catalyst?
B
F and G
Which species cannot appear in the rate expression for scheme 1?
Which is the rate-determining step in scheme 3?
Second one

![The Rate-determining Step
NO2 + CO NO + CO2
rate = k[NO2]2
What can we work out if we are told the following](https://screenshots.scribd.com/Scribd/252_100_85/189/172428533/6.jpeg)