Professional Documents
Culture Documents
Ramon Lu Gerico Alforja Lorenzo Aramil
Uploaded by
ramonlu05Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ramon Lu Gerico Alforja Lorenzo Aramil
Uploaded by
ramonlu05Copyright:
Available Formats
Ramon Lu
Gerico Alforja
Lorenzo Aramil
Chemical Kinetics
Chemical kinetics - speed or rate at which a reaction
occurs
How are rates of reactions affected by
Reactant concentration?
Temperature?
Reactant states?
Catalysts?
The Instantaneous Reaction Rate
Consider the following reaction
A + B C
Define the instantaneous rate of consumption of
reactant A, v
A
| |
dt
A d
A
= v
Reaction Rates and Reaction
Stoichiometry
Look at the reaction
O
3
(g) + NO(g) NO
2
(g) + O
2
(g)
| |
dt
]
O
d[
+ =
dt
]
NO
d[
+ =
dt
d[NO]
- =
dt
O
d
- = rate
2 2 3
Another Example
2 NOCl (g) 2 NO + 1 Cl
2
(g)
| |
dt
d[Cl
+ =
dt
d[NO]
2
1
=
dt
NOCl d
2
1
- = rate
2
]
+
WHY? 2 moles of NOCl disappear
for every 1 mole Cl
2
formed.
The General Case
a A + b B c C + d D
rate = -1 d[A] = -1 d[B] = +1 d[C] = +1 d[D]
a dt b dt c dt d dt
Why do we define our rate in this way?
removes ambiguity in the measurement of reaction rates
Obtain a single rate for the entire equation, not just for
the change in a single reactant or product.
Alternative Definition of the Rate
Rate of conversion related to the
advancement of the reaction, .
dt
d
V
1
= reaction of rate
V = solution volume
An Example
Examine the following reaction
2 N
2
O
5
(g) 4 NO
2
(g) + O
2
(g)
N
2
O
5
NO
2
O
2
Initial n
0 0
Change -2 +4 +
Final n
- 2 4
The N
2
O
5
Decomposition
| | | |
| |
| |
dt
V d
=
dt
] d[O
dt
V d
4 =
dt
] d[NO
dt
V d
2 - =
dt
O N d
2
2
5 2
+
dt
d
V
1
= reaction of rate
Note constant
volume system
The Rate Law
Relates rate of the reaction to the
reactant concentrations and rate
constant
For a general reaction
a A + b B + c C d D + e E
z y x
[C] [B] k[A]
dt
d
V
1
v Rate = = =
Rate Laws (Contd)
The only way that we can determine the
superscripts (x, y, and z) for a non-
elementary chemical reaction is by
experimentation.
Use the isolation method (see first year
textbooks).
For a general reaction
E x + y + z = reaction order
e.g. X = 1; Y = 1; Z = 0
2nd order reaction (x + y + z = 2)
X = 0; Y = 0; Z = 1 (1st order reaction)
X = 2; Y = 0; Z = 0 (2nd order)
Rate Laws for Multistep Processes
Chemical reactions generally proceed via
a large number of elementary steps - the
reaction mechanism
The slowest elementary step the rate
determining step (rds).
An Example Reaction Mechanism
O atoms are intermediates in the above reaction
sequence.
Intermediates generally small, indeterminate
concentrations.
+
+
1
1
2
3 2
3 2
O O O
O O 2O
k
k
k
Integrated Rate Laws
The rate law gives us information about
how the concentration of the reactant
varies with time
How much reactant remains after
specified period of time? Use the
integrated rate laws.
First Order Reaction
A product
Rate = v = - d[A]/d t = k[A]
How does the concentration of the
reactant depend on time?
kt
A
A
o
=
|
|
.
|
\
|
ln
k has units of s
-1
The Half-life of a First Order
Reaction
For a first order reaction, the half-life t
1/2
is calculated as follows.
k
693 0
t
2 1
.
/
=
Radioactive Decay
Radioactive Samples decay according to first
order kinetics.
This is the half-life of samples containing
e.g.
14
C ,
239
Pu,
99
Tc.
Example
|
0
1
14 14
N C
+
Second Order Reaction
A products v = k[A]
2
A + B products v = k[A][B]
Case 1 is 2
nd
order in A
Case 2 is 1
st
order in A and B and 2
nd
order overall
The Dependence of Concentration
on Time
For a second order process where v =
k[A]
2
| | | |
kt
A
1
A
1
o
+ =
Half-life for This Second Order
Reaction.
[A] at t = t = [A]
0
0
2 1
2 1
o 0
A k
1
=
t
or
kt
+
A
1
=
/2 A
1
] [
] [ ] [
/
/
Other Second Order Reactions
Examine the Case 2 from above
A + B products v = k[A][B]
| |
| |
| |
| |
| | | | ( )
o o
o
o
A B kt
A
A
B
B
=
ln
A Pseudo-first Order Reaction
Example hydration of methyl iodide
CH
3
I(aq) + H
2
O(l) CH
3
OH(aq) + H
+
(aq) +
I
-
(aq)
Rate = k [CH
3
I] [H
2
O]
Since we carry out the reaction in aqueous
solution
[H
2
O] >>>> [CH
3
I] [H
2
O] doesnt change
by a lot
Pseudo-first Order (contd)
Since the concentration of H
2
O is
essentially constant
v = k [CH
3
I] [constant]
= k` [CH
3
I] where k` = k [H
2
O]
The reaction is pseudo first order since it
appears to be first order, but it is actually a
second order process.
Sequential First Order Reactions
Suppose we have two first order reactions
occurring in sequence.
v k A
v k A
b a
c b
A B
B C
= (
= (
What is Our Intermediate?
B is an intermediate in the above reaction
sequence.
Clearly B is formed in the first elementary step of
the reaction mechanism and consumed in the final
step.
The Concentration Dependencies
of the Species
The amounts of the reactants are related to the
reaction rates as follows.
( (
= = ( (
(
= ( (
;
a b
a b
d A d C
k A k B
dt dt
d B
k A k B
dt
Sequential Reactions (3)
For a set of initial conditions [A]
o
0, and [B]
o
=
[C]
o
=0 mol/L.
{ }
k
k
A A
A
k A k B
a
a
t
O
t
a b
O
e
d
e
dt
= ( (
(
=
( (
=
Sequential Reactions (4)
The concentration of the intermediate can be
written
{ }
k k
k
B A
k k
a b
t t
a
O
b a
e e
= ( (
Sequential Reactions (5)
k k
k k
C 1 A
k k
b a
t t
a b
O
b a
e e
| |
= + ( (
|
|
\ .
o
o
Note A A B C
Hence C A A B
= + + ( ( ( (
= ( ( ( (
The Rate Determining Step of the
Reaction
What happens if one of the steps in the reaction is
much slower than the other reaction step?
( )
k k
k
k k
lim lim 1 A
k k
1 A
b a
b b
a
t t
a b
O
k k
b a
t
O
e e
C
e
| |
= + ( (
| `
|
\ .
)
= (
Note assuming k
a
<<< k
b
The Rate Determining Step of the
Reaction (2)
If k
b
<<< k
a
( )
k k
k
k k
lim lim 1 A
k k
1 A
b a
a a
b
t t
a b
O
k k
b a
t
O
e e
C
e
| |
= + ( (
| `
|
\ .
)
= (
Temperature Dependence of
Reaction Rates
Reaction rates generally increase with increasing
temperature.
Arrhenius Equation
RT
E
a
e A k
=
A = pre-exponential factor
E
a
= the activation energy
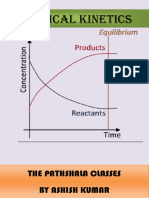
Reactions Approaching Equilibrium
Examine the concentration profiles for the
following simple process.
A B
Time
C
o
n
c
e
n
t
r
a
t
i
o
n
A
B
Approaching Equilibrium
Calculate the amounts of A and B at
equilibrium.
| | | |
k
v
V
A A
eq A
o eq
,
=
|
.
|
\
|
=
| |
/
,
k
v
V
B
eq B
eq
=
|
.
|
\
|
=
The Equilibrium Condition
At equilibrium, v
A,eq
= v
B,eq
.
| | | |
eq B eq eq A eq
v B k v A k
,
/
,
= = =
| |
| |
K
A
B
k
k
eq
eq
= =
/
Elementary steps and the
Molecularity
Kinetics of the elementary step depends only
on the number of reactant molecules in that
step!
Molecularity the number of reactant
molecules that participate in elementary
steps
The Kinetics of Elementary Steps
For the elementary step
unimolecular step
For elementary steps involving more than one
reactant
a bimolecular step
| | A k v products A =
| || | B A k v products B A = +
The Kinetics of Elementary Steps
For the elementary step
unimolecular step
For elementary steps involving more than one
reactant
a bimolecular step
| | A k v products A =
| || | B A k v products B A = +
For the step
a termolecular (three molecule) step.
Termolecular (and higher) steps are not
that common in reaction mechanisms.
| | | | B A k v products B A 2
2
= +
The Steady-State Approximation
Examine the following simple reaction mechanism
| | B k v
2 p
=
Rate of product formation, v
p
, is proportional
to the concentration of an intermediate.
1 2
1
k k
k
A B P
Applying the Steady State
Approximation (SSA)
Look for the intermediate in the mechanism.
Step 1 B is produced.
Reverse of Step 1 B is consumed.
Step 2 B is consumed.
| |
| | | | | | B k B k A k
dt
B d
2 1 1
=
The SSA (Contd)
The SSA applied to the intermediate B.
| |
| | | | | |
| |
| |
2 1
1
2 1 1
k k
A k
B
B k B k A k
0
dt
B d
+
=
+ =
=
SSA The Final Step
Substitute the expression for the concentration of
B into the rate law v
p
.
| |
| |
| |
| | A
k k
k k
dt
P d
B k
dt
P d
2 1
1 2
2
+
=
=
Competing Reactions
Imagine a reaction with a competing side reaction.
= (
= (
v k A
v k A
b a
c b
A B
A C
The Reaction Yield
We can calculate the amount of material
produced from the competing reactions.
J
n
n
k
k
u =
k
J
= the rate constant for the reaction J.
Activated Complex Theory
Consider the following bimolecular reaction
k
A B C +
Presume that the reaction proceeds through the
transition state?
1
1
k
k
k
A B AB P
+
The Activated Complex
The species temporarily formed by the reactant
molecules the activated complex.
A small fraction of molecules usually have the
required kinetic energy to get to the transition state
The concentration of the activated complex is
extremely small.
Transition State Theory (TST)
TST pictures the bi-molecular reaction proceeding
through the activated complex in a rapid-pre-
equilibrium.
1
1
k
k
k
A B AB P
d P
k AB
dt
+
(
(
=
TST (II)
From the thermodynamic equilibrium constant for the
formation of AB
;
A B
AB
A B
A B
AB
p
K
p p
p RT A p RT B
p RT AB
RT
AB K
P
=
= = ( (
(
=
(
= ( (
TST (IV)
Assume that we can obtain the Gibbs energy of
activation from K
.
2
ln
G
B
RT
o
G RT K
k T RT
k e
h P
A
A =
=
The Eyring Equation!
TST (V)
Hence, substituting for the Gibbs energy of activation
in terms of the enthalpy and entropy of activation.
2
S H
B
RT R
o
G H T S
k T RT
k e e
h P
A A
A = A A
=
TST (VI)
Expressing the enthalpy of activation in terms of the
activation energy.
2 bimolcular gas phasereaction
unimolecular gas phase
or solutionreaction
a
a
H E RT
H E RT
A =
A =
TST (VII)
The final result for a bimolecular gas phase reaction.
2
2
a
E
S
B
RT R
o
k T RT
k e e e
h P
A
=
2
2
2
a a
E E
S
B
RT R RT
o
S
B
R
o
k T RT
k Ae e e
h P
k T RT
A e
h P
A
+
A
+
= =
=
TST (VIII)
The final result for a unimolecular gas phase reaction
(or any solution reaction).
a
E
S
B
RT R
r
o
k T RT
k e e e
h P
A
=
1
1
a a
E E
S
B
RT R RT
r
o
S
B
R
o
k T RT
k Ae e e
h P
k T RT
A e
h P
A
+
A
+
= =
=
You might also like
- 2.kinetics Homogenous ReactionsDocument33 pages2.kinetics Homogenous ReactionsArief Al Imam HidayatullahNo ratings yet
- Physical Chemistry - KineticsDocument66 pagesPhysical Chemistry - KineticsarieleliannasternNo ratings yet
- Content Marketed & Distributed By: Chemical KineticsDocument9 pagesContent Marketed & Distributed By: Chemical KineticsPrithviraj NetkeNo ratings yet
- Week 2. Chemical Kinetics Analysis of Rate EquationDocument31 pagesWeek 2. Chemical Kinetics Analysis of Rate EquationYuni ApriyaniNo ratings yet
- Reaction KineticsDocument37 pagesReaction KineticsNurshuhada NordinNo ratings yet
- Chem Chapt13 PractiseDocument5 pagesChem Chapt13 PractiseqwerNo ratings yet
- X. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsDocument28 pagesX. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsJon Bisu DebnathNo ratings yet
- Reaction KineticsDocument26 pagesReaction Kineticsdimasaditya28No ratings yet
- Chemistry Unit 5.4Document8 pagesChemistry Unit 5.4Sonal PereraNo ratings yet
- Unit 15 Kinetics: ChemicalDocument75 pagesUnit 15 Kinetics: Chemicalpetercyh175No ratings yet
- Chemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, CanadaDocument34 pagesChemical Kinetics: Chung (Peter) Chieh Professor of Chemistry University of Waterloo Waterloo, Ontario, Canadadescar84No ratings yet
- Chapter 2b KineticsDocument11 pagesChapter 2b KineticsSankar SasmalNo ratings yet
- Chemical KineticsDocument134 pagesChemical Kineticstapas kundu100% (1)
- Unit-7 Chemical Kinetics 2023Document13 pagesUnit-7 Chemical Kinetics 2023jagannathanNo ratings yet
- Kinetics IntroductionDocument55 pagesKinetics IntroductionAgano juma mwakasendoNo ratings yet
- Chemical Engineering KineticsDocument45 pagesChemical Engineering KineticsMelissa Marie DimaculanganNo ratings yet
- Chemical KineticsDocument41 pagesChemical Kineticskishangopi1230% (1)
- Chapter 4Document32 pagesChapter 4Nguyen NhatNo ratings yet
- For Chapter 5555Document58 pagesFor Chapter 5555bahru demekeNo ratings yet
- Zumdahl Chemical Kinetics NotesDocument6 pagesZumdahl Chemical Kinetics NotesMalletNjonkemNo ratings yet
- Chemical KineticsDocument56 pagesChemical KineticsMohamed KhaledNo ratings yet
- Chemical KineticsDocument3 pagesChemical KineticsRachel AustriaNo ratings yet
- Chemical Kinetics-3Document17 pagesChemical Kinetics-3Ashutosh KunwarNo ratings yet
- Chap 8 Reaction Kinetics 1415FARRADocument129 pagesChap 8 Reaction Kinetics 1415FARRA黄麒安No ratings yet
- 1.0 Reaction KineticsDocument142 pages1.0 Reaction KineticsKhairul Aswari Ab RahmanNo ratings yet
- Chemistry Pre-U Chemistry Sem 1 Chap 5 PDFDocument85 pagesChemistry Pre-U Chemistry Sem 1 Chap 5 PDFJIANHUI0160% (1)
- Lection 5 (Eng) PDFDocument9 pagesLection 5 (Eng) PDFa320neoNo ratings yet
- Fe Chemical EngineeringDocument5 pagesFe Chemical EngineeringJudith LugoNo ratings yet
- Chemical Kinetics-12Document18 pagesChemical Kinetics-12Manas ChhabraNo ratings yet
- Chapter 16 (Kinetics)Document9 pagesChapter 16 (Kinetics)Richard KimNo ratings yet
- Chemical Kinetics1Document59 pagesChemical Kinetics1farooq_bagbanNo ratings yet
- Laju Reaksi PDFDocument9 pagesLaju Reaksi PDFHafsemi RapsanjaniNo ratings yet
- Chemical KineticsDocument72 pagesChemical KineticsSiddhartha KumarNo ratings yet
- Experiment 4Document30 pagesExperiment 4Mohsen Haj HassanNo ratings yet
- Physical Chemistry 2 Midterm Note I. Reaction Kinetic:: 1. Some ConceptsDocument9 pagesPhysical Chemistry 2 Midterm Note I. Reaction Kinetic:: 1. Some ConceptsTrung VõNo ratings yet
- Reaction MechanismsDocument17 pagesReaction MechanismskimNo ratings yet
- Topic 6 & 16: KineticsDocument57 pagesTopic 6 & 16: Kineticsapi-546066323No ratings yet
- Rates of Chemical Reactions: Kinetics 1Document68 pagesRates of Chemical Reactions: Kinetics 1K CabeguinNo ratings yet
- Mod 4 Revision Guide 1 Reaction Kinetics AQA A2 ChemistryDocument5 pagesMod 4 Revision Guide 1 Reaction Kinetics AQA A2 Chemistryviyas07No ratings yet
- Department of Chemistry: Course No.: CH 101Document14 pagesDepartment of Chemistry: Course No.: CH 101liz_hobbs79No ratings yet
- Chemistry Form 6 Chap 05 NewDocument83 pagesChemistry Form 6 Chap 05 Newmusafir24No ratings yet
- DP Chemical KineticsDocument32 pagesDP Chemical KineticsAniket RayNo ratings yet
- Chemical Kinietics PDFDocument19 pagesChemical Kinietics PDFYoNo ratings yet
- Skf3023 Lecture 1Document33 pagesSkf3023 Lecture 1NOR SANISAH BINTI ARSADNo ratings yet
- Chemical Kinetics: H + I 2 HI H I HIDocument49 pagesChemical Kinetics: H + I 2 HI H I HIputriprastyarNo ratings yet
- Material Balance With Chemical ReactionDocument50 pagesMaterial Balance With Chemical ReactionKunal AgarwalNo ratings yet
- Kinetics Handout - 16.10.20 (Chemistry)Document10 pagesKinetics Handout - 16.10.20 (Chemistry)Jayjeet ChakrabortyNo ratings yet
- ws14 1Document6 pagesws14 1Evilasio CostaNo ratings yet
- ws14 1Document6 pagesws14 1Evilasio CostaNo ratings yet
- The Rate of ReactionDocument35 pagesThe Rate of ReactionMadhav SethiaNo ratings yet
- Notes Chemical KineticsDocument17 pagesNotes Chemical KineticsAMAR KUMARNo ratings yet
- Environmental Engineering Unit OperationsDocument40 pagesEnvironmental Engineering Unit OperationsselamitspNo ratings yet
- Chemical KineticsDocument22 pagesChemical Kineticspragun0507No ratings yet
- ws14 1Document6 pagesws14 1Diana Jean Alo-adNo ratings yet
- Chemical and Enzyme Kinetics Lecture 2Document47 pagesChemical and Enzyme Kinetics Lecture 2downdstairs45No ratings yet
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- NSTP 1st Sem PPT PovertyDocument6 pagesNSTP 1st Sem PPT Povertyramonlu05No ratings yet
- Citation GuideDocument6 pagesCitation Guideramonlu05No ratings yet
- Nursing Health AssessmentDocument6 pagesNursing Health Assessmentramonlu05100% (1)
- Gen PathDocument3 pagesGen Pathramonlu05No ratings yet
- 15 Chem KinetDocument51 pages15 Chem KinetRoua Ali100% (2)
- King Arthur - BackgroundDocument12 pagesKing Arthur - Backgroundramonlu05No ratings yet
- G10 - Handout - Organic - Makeup Handout - First WeekDocument4 pagesG10 - Handout - Organic - Makeup Handout - First WeekSheela BatterywalaNo ratings yet
- Sales Contract Bangladesh Complete and Signed by Jolly 22 July 2021Document18 pagesSales Contract Bangladesh Complete and Signed by Jolly 22 July 2021Fantania BerryNo ratings yet
- Reduction of Environmental ImpactDocument16 pagesReduction of Environmental ImpactJohn Vincent MalvarNo ratings yet
- Filtration of AluminiumDocument218 pagesFiltration of AluminiumNico Agung NugrahaNo ratings yet
- Application of ESP For Gas Cleaning in Cement Industry - With Reference To IndiaDocument24 pagesApplication of ESP For Gas Cleaning in Cement Industry - With Reference To IndiaSJ ChuaNo ratings yet
- EagleBurgmann MG1 enDocument4 pagesEagleBurgmann MG1 ensanjeevvangeNo ratings yet
- PR-1154 - Gas Testing ProcedureDocument28 pagesPR-1154 - Gas Testing ProcedureRAHULNo ratings yet
- 51314-3985-Methanol-Induced Internal Stress CorrosDocument18 pages51314-3985-Methanol-Induced Internal Stress CorrosMahmoud GamalNo ratings yet
- WPS - PQR (Sa516 GR.70)Document4 pagesWPS - PQR (Sa516 GR.70)miltonangulomorrisNo ratings yet
- Waste-To-Energy Plant Process Safety ChallengesDocument5 pagesWaste-To-Energy Plant Process Safety Challengessomesh sharmaNo ratings yet
- Materials and Design: Ehab A. El-Danaf, Magdy M. El-Rayes, Mahmoud S. SolimanDocument6 pagesMaterials and Design: Ehab A. El-Danaf, Magdy M. El-Rayes, Mahmoud S. Solimankamal touilebNo ratings yet
- Raw Materials-IronDocument22 pagesRaw Materials-IronAilson Silva AlvesNo ratings yet
- Specifications: Customer Item Model Name Part No DateDocument11 pagesSpecifications: Customer Item Model Name Part No Datejoroma58No ratings yet
- Cut Diet Lean MassDocument62 pagesCut Diet Lean Masspakzeeshan167% (3)
- Effect of Transition Metal Oxides On Decomposition and Deflagration of Composite Solid Propellant Systems: A SurveyDocument8 pagesEffect of Transition Metal Oxides On Decomposition and Deflagration of Composite Solid Propellant Systems: A SurveyAmin AminiNo ratings yet
- Antinociceptive Activity of Buddleja Globosa (Matico)Document6 pagesAntinociceptive Activity of Buddleja Globosa (Matico)alinumlNo ratings yet
- Tooth DecayDocument28 pagesTooth DecayRyan Carlo CondeNo ratings yet
- REPORTDocument31 pagesREPORTUnique Boss50% (2)
- Work Instructions (W.I.)Document18 pagesWork Instructions (W.I.)Shamsul Azhar MohdNo ratings yet
- InternshipDocument16 pagesInternshipSarthak SinghNo ratings yet
- Redox Regulation, Thioredoxins, and Glutaredoxins (Review 2023)Document15 pagesRedox Regulation, Thioredoxins, and Glutaredoxins (Review 2023)Hatem BoubakriNo ratings yet
- 4th Health2 For Demo Explicit TeachingDocument4 pages4th Health2 For Demo Explicit TeachingLeony Cipriano100% (2)
- 409 Data BulletinDocument12 pages409 Data BulletinWilliam PaivaNo ratings yet
- HW 03 On IUPAC NamingDocument1 pageHW 03 On IUPAC NamingEMERALDARCANISTNo ratings yet
- Handbook of Carbon Nanotubes Polymer NanDocument182 pagesHandbook of Carbon Nanotubes Polymer NanMario Allesina JuniorNo ratings yet
- The 7 TH International Conference On Unsaturated Soils (UNSAT2018)Document7 pagesThe 7 TH International Conference On Unsaturated Soils (UNSAT2018)pooNo ratings yet
- Rotary PumpsDocument31 pagesRotary PumpsalbertNo ratings yet
- Hyperbaric Oxygen TherapyDocument7 pagesHyperbaric Oxygen Therapy18juni1995No ratings yet
- Nursing Care Plan PrenatalDocument5 pagesNursing Care Plan PrenatalKim Galamgam100% (2)
- Householders Guide To Flat RoofingDocument24 pagesHouseholders Guide To Flat RoofingBudi SudrajatNo ratings yet