0% found this document useful (0 votes)
2K views58 pagesLiving Polymerization
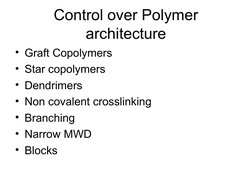
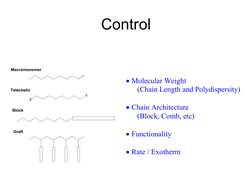
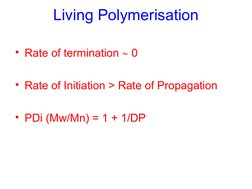
The document discusses living polymerization and controlled radical polymerization techniques such as atom transfer radical polymerization (ATRP). It explains that living polymerizations allow control over the polymer architecture, molecular weight, and molecular weight distribution. Specifically, it notes that living polymerizations have a constant number of polymer chains, no permanent chain termination, and result in narrow molecular weight distributions. It provides examples of using ATRP to synthesize polymers with controlled molecular weights, block copolymers, star polymers, and end-functionalized polymers.
Uploaded by
dohuucauCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
2K views58 pagesLiving Polymerization
The document discusses living polymerization and controlled radical polymerization techniques such as atom transfer radical polymerization (ATRP). It explains that living polymerizations allow control over the polymer architecture, molecular weight, and molecular weight distribution. Specifically, it notes that living polymerizations have a constant number of polymer chains, no permanent chain termination, and result in narrow molecular weight distributions. It provides examples of using ATRP to synthesize polymers with controlled molecular weights, block copolymers, star polymers, and end-functionalized polymers.
Uploaded by
dohuucauCopyright
© Attribution Non-Commercial (BY-NC)
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
- Living Polymerization
- Polymers in Everyday Use
- Control over Polymer Architecture
- Control
- Test for Living Polymerisation
- Living Polymerisation Types
- Characteristics of Living Polymerisation
- Living Systems
- ATRP
- Living and Controlled Polymerisations
- ATRP Applications
- Hyperbranched Polymers
- Synthesis of End-functionalized Polymers
- Challenges for ATRP
- Supported Catalysts
- What Can Living Polymerizations Do?
- Synthesis, Characterization and Application
- Materials
- Synthesis of PDMAEMA Brushes


![Test for Living Polymerisation
0
20
40
60
80
100
Mn
% Conversion
kp[Pol*]
ln [M]0/[M]
time](https://screenshots.scribd.com/Scribd/252_100_85/189/28374801/5.jpeg)



![Rate of Initiation
= ki[I][M]
Rate of Propagation = kp[M*][M]
[M*] = [I]
Integration leads to,
ln[M]0/[M] = kp[M*]t
As t](https://screenshots.scribd.com/Scribd/252_100_85/189/28374801/10.jpeg)

























































































