Professional Documents
Culture Documents
Iron - Carbon System: 1 Mechanical Engineering, SOT, KIIT University
Iron - Carbon System: 1 Mechanical Engineering, SOT, KIIT University
Uploaded by
Chandra SekharOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Iron - Carbon System: 1 Mechanical Engineering, SOT, KIIT University
Iron - Carbon System: 1 Mechanical Engineering, SOT, KIIT University
Uploaded by
Chandra SekharCopyright:
Available Formats
Module 3
Iron – Carbon System
Mechanical Engineering, SOT, KIIT
1
University
Equilibrium Diagrams
Mechanical Engineering, SOT, KIIT
2
University
Definition & Basic Concepts
Component: substances whose presence is necessary & sufficient
to make a system (elements / compounds present in mixture)
Phase: physically & chemically homogeneous portion of a system
separated by interface, i.e. pure material, solid, liquid, gas
System: whole complex of phases of one or several components at
different pressures, composition & temperatures, e.g. Fe-C system
Homogeneous System: contains a single phase, e.g. liquid solution
Heterogeneous System: contains two or more phases , e.g. co-
existence of liquid and its crystals is a two phase system
Mechanical Engineering, SOT, KIIT
3
University
Phase Boundary: interface between different phases across,
which the physical and / or chemical properties changes abruptly,
e.g. sugar-water, water-ice, FCC iron-BCC iron
Solid-Solution: consists of atoms at least two different types; the
solute atoms occupy either substitutional or interstitial position
in the solvent lattice and the crystal structure of solvent is
maintained
Solubility Limit: maximum concentration of solute atoms that may
dissolve in the solvent to form a solid solution
Mechanical Engineering, SOT, KIIT
4
University
(a). Three forms of water –
gas, liquid & solid – are each
a phase
(b). Water & alcohol have
unlimited solubility
(c). Salt & water have limited
solubility
(d). Oil & water have virtually
no solubility
Illustration of Phases & Solubility
Mechanical Engineering, SOT, KIIT
5
University
Equilibrium: system is at equilibrium, if its free energy is at
minimum under some specified combination of temperature,
pressure, and composition, e.g. characteristics of the system
do not change with time and system is therefore stable
Phase Equilibrium: refers to an equilibrium state to a system
containing two or more phases, in which the characteristics of
phases do not change with time
Mechanical Engineering, SOT, KIIT
6
University
Equilibrium Diagram / Phase Diagram / Constitutional Diagram
Defines the regions of stability of phases that can occur in an
alloy system under different conditions of temperature &
composition (at constant pressure)
Temperature & pressure are two external factors determining the
state of system, but in case of metals, the effect of pressure is
neglected (there is only one external factor)
Coordinates of the diagram are temperature (ordinate, y-axis)
and composition (abscissa, x-axis)
Only in case of single component phase diagram, variables
temperature & pressure are used as shown in the figure
Mechanical Engineering, SOT, KIIT
7
University
Liquid
Phase
Solid
Phase
Pressure
Gaseous
Phase
Temperature
Single Component Phase Diagram
Mechanical Engineering, SOT, KIIT
8
University
Phase Diagram enables following:
Average composition of components in the alloy at any
temperature
Phase transformation to be followed during heating or cooling
of the alloy under equilibrium conditions, i.e. when all
processes in given system are reversible
Phase content of the alloy at any temperature & average
composition by Lever Rule
Composition of the components in different phases at any
temperature & average composition by Lever Rule
Mechanical Engineering, SOT, KIIT
9
University
Phase Rule
Establishes the relationship between the No. of Degrees of
Freedom, the No. of Components and the No. of phases
present in the alloy system
All changes taking place in the alloy system in accordance with
the external conditions conform to this rule
Mathematically,
F = (C + n – P) = (C + 1 – P)
F: No. of Degrees of Freedom
C: No. of Components
n: No. of external factors (temp. & press.) = 1
P: No. of Phases in equilibrium
Mechanical Engineering, SOT, KIIT
10
University
Degrees of Freedom
No. of independent external or internal factors (temperature,
pressure & composition), which may be altered without causing
the formation of a new phase or disappearance of an existing
phase in the system
Pure metal at One component, two phase F=0
solidification temp. system (C = 1 & P = 2)
Non-Variant System
Alloy of two metals Two component, two phase F=1
at the start of system (C = 2 & P = 2)
Mono-Variant
solidification
System
F = 2 is a Divariant system, where system may be at equilibrium at
different tempertaures and composition
Mechanical Engineering, SOT, KIIT
11
University
Isomorphous Alloy System
Complete solid solubility for all compositions and having
same homogeneous crystal structure for all ratios of the
components, i.e. Components are completely soluble in the
liquid & solid states
Example: Copper – Nickel alloy
Essential Conditions:
Components should have same type of crystal
Sizes of atoms should be very similar (size difference
should not exceed more than 8 %)
Alloys forming homogeneous solid solution like isomorphous
alloy system are widely used as engineering materials
Mechanical Engineering, SOT, KIIT
12
University
Isomorphous Alloy System, e.g. Cu-Ni alloy system
Mechanical Engineering, SOT, KIIT
13
University
Lever Rule
Important relationship applied to any two-phase region of a two-
component (Binary) phase diagram, which aids in following:
• Determination of the composition of two phases
• Determination of the relative amount of the two phases
Tie Line is constructed across the two-phase region at a
particular temperature of alloy under consideration and average
alloy composition is located on it
Fraction of one phase is computed by taking the length of tie
line from average alloy composition to phase boundary for other
phase and dividing it by the total tie line length
Mechanical Engineering, SOT, KIIT
14
University
Fraction of other phase is determined in the same manner
Each phase is multiplied by 100, If phase percentages are
desired
If composition axis is scaled in wt. %, the phase fractions
computed are mass (or weight) of a specific phase divided by
the total alloy mass (or weight)
Mass of each phase is computed from the product of each
fraction and total alloy mass
Mechanical Engineering, SOT, KIIT
15
University
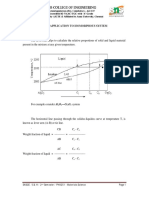
How to construct a Tie Line
1. Tie line is constructed across the two-phase region at
temperature of alloy
2. Intersections of tie line and phase boundaries on either side
are noted
3. Perpendiculars are dropped from these intersections to
horizontal composition axis from which composition of each
of respective phases is noted
Mechanical Engineering, SOT, KIIT
16
University
Consider an alloy corresponding
to point ‘Y’ (temp. – 1200 oC &
average composition of 73 % Cu)
having both solid & liquid phases
Temperature (oC)
According to Tie Line, the solid
phase in the alloy sample has 62
% Cu and the liquid phase in the
alloy sample has 78 % Cu
Assume the alloy sample to be of
100 gms, then wt. of Cu and Ni in
62 % 78 %
73 % the sample are 73 gms and 27
gms respectively
Composition % Cu
Mechanical Engineering, SOT, KIIT
17
University
Assume wt. of solid phase in alloy sample to be ‘w’
Therefore, wt. of Cu in the solid and liquid phases will be ‘0.68
w’ and ‘0.78 (100-w)’ respectively
Also, the total wt. of Cu in the alloy = Sum of the wts. of Cu in
solid and liquid phases
or 73 = 0.62 w + 0.78 (100-w)
or 78 – 73 = (0.78 – 0.62) w
or w/100 = [(0.78 – 0.73) / (0.78 – 0.62)]
Hence, wt. % solid = (b / c)
& wt. % liquid = (a / c)
Mechanical Engineering, SOT, KIIT
18
University
Eutectic Alloy System
Types:
(Type I): Components are completely soluble in the liquid
state but are insoluble in each other in the solid state
(Type II): Components are completely soluble in the liquid
state but partially soluble in solid state
Example: Tin-Zinc alloy, Cadmium-Bismuth alloy
Main Characteristics: (Eutectic Point)
Eutectic composition & temperature
Eutectic reaction
cooling
Liqeutectic (α + )eutectic
heating
Mechanical Engineering, SOT, KIIT
19
University
Construction of Eutectic Alloy System (Type – I)
Mechanical Engineering, SOT, KIIT
20
University
- solid solution of ‘B’ in ‘A’
- solid solution of ‘A’ in ‘B’
Eutectic Alloy System (Type – I)
Mechanical Engineering, SOT, KIIT
21
University
Eutectic Alloy System (Type – II)
Mechanical Engineering, SOT, KIIT
22
University
Peritectic Alloy System
Components have complete mutual solubility in liquid state
and limited solubility in solid state
it differs from the eutectic alloy system (Type-II), as the
crystals of -solid solution precipitated at the beginning of
solidification reacts with liquid alloy of definite composition
to form new crystals of -solid solution
Example: Fe-C alloy
Main Characteristics: Reaction occurs at constant
temperature
Mechanical Engineering, SOT, KIIT
23
University
Peritectic Alloy System
Mechanical Engineering, SOT, KIIT
24
University
Fe – C Equilibrium Diagram
Mechanical Engineering, SOT, KIIT
25
University
Free Energy Curve of Iron
Mechanical Engineering, SOT, KIIT
26
University
Allotropic Forms of Iron
Mechanical Engineering, SOT, KIIT
27
University
• Two allotropic forms of Fe: -iron (BCC, 2.86 Å) & -iron (FCC, ,
3.63 Å)
• -iron exists up to temp. 910 oC & inbetween 1400 oC to 1539 oC
(910 oC and 1400 oC are the intersection points of two curves)
• From 768 oC to 910 oC, -iron is referred as ()-iron and is
paramagnetic (magnetically weak) in behavior, i.e. from 768 oC
onwards iron changes its behavior from ferromagnetic
(magnetically strong) to paramagnetic
• From 1400 oC to 1539 oC, -iron is referred as ()-iron, i.e. from
1400 oC onwards iron changes its allotropic form from FCC to
BCC structure
• Between 910 oC to 1400 oC, iron exists as -iron with FCC
structure
Mechanical Engineering, SOT, KIIT
28
University
Simplified Fe-C Equilibrium Diagram
Mechanical Engineering, SOT, KIIT
29
University
• Critical points along line GS are designated as A3, i.e. Ac3 is for
heating & Ar3 is for cooling
• Critical points along line SE are designated as Acm (to
distinguish secondary / pro-eutectoid cementite, i.e. Fe3C II
• Critical points along line PSK are designated as A1, i.e. Ac1 is for
heating & Ar1 is for cooling
Nomenclature:
A: Arrest
C: Chauffage (heating)
R: Refroidissement (cooling)
Mechanical Engineering, SOT, KIIT
30
University
• Fe-C alloys having carbon percent up to 2.0 % are Steels
• Fe-C alloys having carbon percent more than 2.0 % & less than
6.67 % are Cast Irons (CI)
• Solid solution of carbon in -iron: Ferrite
• Solid solution of carbon in -iron: Austenite
• Points ‘A’ & ‘G’ corresponds to allotropic transformation , i.e.
point ‘A’ is for formation of austenite (-iron) & point ‘G’ is for
formation of ferrite (-iron)
• Max. solubility of carbon in -iron is 0.022 % (at 723 oC, i.e. point
‘P’) and 0.0025 % (at 0 oC)
• Max. solubility of carbon in -iron is 2.0 % at 1130 oC (point ‘E’)
Mechanical Engineering, SOT, KIIT
31
University
Iron carbide (Fe3C) / Cementite
• Chemical compound having 6.67 % carbon (constant comp.)
• Orthorhombic lattice with close-packed atoms
• Melting point of 1550 oC & ferromagnetic up to 217 oC
• High hardness , i.e. BHN 700 & low ductility
• Under definite conditions, it can be decomposed to form free
carbon ,i.e. graphite
Allotropic transformation of ()-iron to -iron at high temperatures is
neglected & not shown in the simplified Fe-C diagram, as it is of no
practical use, i.e. ()-iron crystal precipitation from liquid is not shown
and only direct precipitation of -iron crystals from liquid is shown
Mechanical Engineering, SOT, KIIT
32
University
Primary Solidification
Mechanical Engineering, SOT, KIIT
33
University
Primary Solidification
Mechanical Engineering, SOT, KIIT
34
University
• Transformation from liquid to solid state for Fe-C alloys
• Line ‘ACB’ is liquidus line , where liquid alloys begin to solidify
• Line ‘AECF’ is solidus line, where complete solidification of
liquid alloy takes place
• Austenite precipitates along line ‘AC’ & its composition vary
along line ‘AC’, whereas composition of liquid vary along line
‘AE’ (proportion of phases may be obtained by Lever Rule)
• Cementite precipitates along line ‘BC’ having constant
composition of 6.67 % carbon, where as composition of liquid
vary along line ‘BC’ (proportion of phases can be obtained by
Lever Rule)
Mechanical Engineering, SOT, KIIT
35
University
• Point ‘C’ is Eutectic Point with Eutectic Temp. (te) of 1130 oC &
Eutectic Comp. of 4.3 % carbon, where both austenite &
cementite (Ledeburite) precipitates out simultaneously
• All Fe-C alloys having carbon more than 2.0 % (i.e. cast iron) will
completely solidify at 1130 oC (along line ECF) to form eutectic
solid solution, i.e. ledeburite
• Fe-C alloy at line ‘ECF’ having any carbon % will have liquid
phase with composition same as that of eutectic composition, i.e.
along line ‘ECF’, complete liquid phase will form eutectic
ledeburite
• Eutectic Transformation / Reaction:
Liquid Ledeburite (Austenite + Cementite)
Mechanical Engineering, SOT, KIIT
36
University
• C = 2 (Fe & C), P = 3 (Liquid, Austenite & Cementite) & F = 0,
therefore it is a non-variant system / reaction, where all the
three phases have definite carbon comp. at constant temp. of
1130 oC, i.e. liquid = 4.3 %, eutectic ledeburite (austenite = 2.0
% & cementite = 6.67 %)
• Fe-C alloy with carbon % more than 2.0 & less than 4.3 are
known as ‘Hypo-Eutectic’ cast iron and consists of austenite
and eutectic ledeburite
• Fe-C alloy with carbon % more than 4.3 are known as ‘Hyper-
Eutectic’ cast iron and consists of eutectic ledeburite and
primary cementite
Mechanical Engineering, SOT, KIIT
37
University
Secondary Solidification
Mechanical Engineering, SOT, KIIT
38
University
Secondary Solidification
Mechanical Engineering, SOT, KIIT
39
University
• Transformation from solid to solid state for Fe-C alloys
• Allotropic transformation of -iron to -iron, as well as
decomposition of austenite into ferrite & cementite (cementite
precipitates as an excess carbon
• Line ‘GS’ indicates beginning of the decomposition of austenite
and precipitation of ferrite
• Critical points along line ‘GS’ are designated as Ac3 in heating
and Ar3 in cooling
• Line ‘SE’ indicates precipitation of excess carbon as cementite,
known as secondary / pre-eutectoid cementite
• Temperatures along line ‘SE’ are designated as Acm points
Mechanical Engineering, SOT, KIIT
40
University
• Line ‘SE’ indicates precipitation of excess carbon as cementite,
known as secondary / pre-eutectoid cementite, which indicates
reduction in the solubility of carbon in -iron (austenite) with
decrease in temperature
• Point ‘S’ corresponds to minimum temp. of 723 oC with 0.8 %
carbon at which austenite exists in a state of equilibrium
• Point ‘S’ is also referred as ‘Eutectoid’ point with Eutectoid
Temp. of 723 oC & Eutectoid Comp. of 0.8 % carbon
• At this point, austenite decomposes with simultaneous
precipitation of ferrite and cementite (secondary cementite), i.e.
eutectoid mixture known as Pearlite
Mechanical Engineering, SOT, KIIT
41
University
• Eutectoid Transformation / Reaction:
Austenite Pearlite(ferrite + cementite)
• C = 2 (Fe & C), P = 3 (austenite, ferrite & cementite) & F = 0,
therefore, it is a non-variant system / reaction
• Line ‘PSK’ indicates complete decomposition of austenite at
constant temperature 723 oC, with critical points at which
pearlite is formed during cooling designated as Ar1 and also
transformation of pearlite to austenite upon heating as Ac1
Mechanical Engineering, SOT, KIIT
42
University
• Pearlite structure consists of thin alternating plates / lamelae
of ferrite and cementite and hence, also referred as Lamellar
Pearlite
• Depending upon heat treatment process, Granular Pearlite
may be formed consisting of rounded globules of cementite in
ferrite field
• Line ‘PQ’ represents in the decrease in the solubility of carbon
in -iron with decrease in the temperature and the excess
carbon precipitates out as ‘Tertiary Cementite’
• Fe-C alloys less than 0.8 % carbon and from 0.8 to 2.0 %
carbon are called as Hypo-Eutectoid & Hyper-Eutectoid steels
Mechanical Engineering, SOT, KIIT
43
University
You might also like
- 2306A Manual de Diagnotico PDFDocument156 pages2306A Manual de Diagnotico PDFMeriem ZAGRIRINo ratings yet
- Electric Snap Circuits 102-305Document76 pagesElectric Snap Circuits 102-305Science HouseNo ratings yet
- Materials For Engineering 20ME11T Unit V - HEAT TREATMENT PROCESSESDocument16 pagesMaterials For Engineering 20ME11T Unit V - HEAT TREATMENT PROCESSESThanmay JS100% (2)
- Alloy & Special SteelsDocument33 pagesAlloy & Special Steelstanishka narayanNo ratings yet
- Phase Rule: Upma Shriavstava Assistant Professor, Deptt of Chemistry Govt V.Y.T.PG. College DurgDocument29 pagesPhase Rule: Upma Shriavstava Assistant Professor, Deptt of Chemistry Govt V.Y.T.PG. College Durgramukaka100% (1)
- Yvoh8 18HP SM 0114 (En) PDFDocument224 pagesYvoh8 18HP SM 0114 (En) PDFTirta Noegraha100% (4)
- Yaris 1nz-Fe (Engine)Document12 pagesYaris 1nz-Fe (Engine)esquisofNo ratings yet
- PhysicalDocument79 pagesPhysicallividiveNo ratings yet
- Chapter 8 Phase Diagrams UpdatedDocument80 pagesChapter 8 Phase Diagrams UpdatedSalman Khalil100% (1)
- Deutz 914 Control BoxDocument6 pagesDeutz 914 Control BoxCarlos GustavoNo ratings yet
- Material Science Solid Solutions: Chapter II - Phase DiagramsDocument24 pagesMaterial Science Solid Solutions: Chapter II - Phase DiagramsPrakash KatdareNo ratings yet
- Installation Operation Maintenance: Cooling Only: CGAN 209-210-211-212-213-214Document40 pagesInstallation Operation Maintenance: Cooling Only: CGAN 209-210-211-212-213-214rlgalo100% (1)
- Reservoir Simulation StudyDocument143 pagesReservoir Simulation StudyKunal Khandelwal0% (1)
- UNIT-5 Phase EquilibriaDocument13 pagesUNIT-5 Phase EquilibriaALOK KUMARNo ratings yet
- Top 10 OU Revised 3.1docxDocument11 pagesTop 10 OU Revised 3.1docxBill ClarkeNo ratings yet
- 06 - Reactor DesignDocument28 pages06 - Reactor DesignNoman AslamNo ratings yet
- AP Chemistry: Chapter 7 - Atomic Structure & PeriodicityDocument14 pagesAP Chemistry: Chapter 7 - Atomic Structure & PeriodicityS. Green100% (1)
- Astm D4809Document9 pagesAstm D4809Kamruzaman MiahNo ratings yet
- DIN EN 50600 Question and AnswersDocument2 pagesDIN EN 50600 Question and AnswersPercy GoitsemangNo ratings yet
- 소재상평형 (조경식)Document93 pages소재상평형 (조경식)윤여춘No ratings yet
- Module - 3, Iron - Carbon SystemDocument43 pagesModule - 3, Iron - Carbon SystemSayaf Khan PathanNo ratings yet
- Engineering Materials 24-26Document31 pagesEngineering Materials 24-26Sanu SouravNo ratings yet
- Chapter 9Document57 pagesChapter 9Pavan PonnadaNo ratings yet
- New Civil Module5 PDF NotesDocument18 pagesNew Civil Module5 PDF NotesDrMohan KumarNo ratings yet
- Chapter 4Document68 pagesChapter 4Ermias GuragawNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- Structural Analysis of Nanomaterials: Lecture 04: Phase Diagram: Determination of PhasesDocument31 pagesStructural Analysis of Nanomaterials: Lecture 04: Phase Diagram: Determination of PhaseswinnieNo ratings yet
- Unit Iv Phase Rule and AlloysDocument15 pagesUnit Iv Phase Rule and AlloysMadhavanIceNo ratings yet
- Phase DiagramsDocument21 pagesPhase DiagramsJnrNo ratings yet
- Chapter 4-Phase DiagramDocument16 pagesChapter 4-Phase Diagramtky96No ratings yet
- Session 5 Topic 5 Isomorphous Systems, The Tie-Line RuleDocument2 pagesSession 5 Topic 5 Isomorphous Systems, The Tie-Line RuleThaya GanapathyNo ratings yet
- Basic Engineering Lecture Program: Physical MetallurgyDocument30 pagesBasic Engineering Lecture Program: Physical Metallurgymakarsk2007No ratings yet
- 6 Phase DiagramDocument22 pages6 Phase DiagramAdiyat AdiNo ratings yet
- Unit-II-Free Energy in Chemical EquilibriaDocument17 pagesUnit-II-Free Energy in Chemical EquilibriaAppu MadanNo ratings yet
- 2-Phase RuleDocument18 pages2-Phase RuleSomesh Ashok BagalNo ratings yet
- Lecture n.15 Phase DiagramsDocument24 pagesLecture n.15 Phase DiagramsMOHAMMAD JAWAD QASIMNo ratings yet
- Phase DiagramsDocument80 pagesPhase DiagramsWilliams AkandiNo ratings yet
- CHAPTER 3 Phase Diagram TTT HT - 1stDocument25 pagesCHAPTER 3 Phase Diagram TTT HT - 1stAriff AziziNo ratings yet
- Unit 5 - Chemistry - WWW - Rgpvnotes.inDocument16 pagesUnit 5 - Chemistry - WWW - Rgpvnotes.inAyush Kr. SharmaNo ratings yet
- MPS8 111923Document59 pagesMPS8 111923joshuaang456No ratings yet
- Tutorial 3 SolutionDocument5 pagesTutorial 3 SolutionUmmi Kalthum MohamadNo ratings yet
- Chapter III Equilibrium DiagramDocument38 pagesChapter III Equilibrium DiagramHoàng Minh LongNo ratings yet
- Assignment No 3 PhysicalDocument7 pagesAssignment No 3 PhysicalTaimoor Hassan KhanNo ratings yet
- Phase Diagrams For Metallic SystemsDocument5 pagesPhase Diagrams For Metallic SystemsZesi Villamor Delos SantosNo ratings yet
- Week 9 10 - 1 - IPE 2203-LecturesDocument86 pagesWeek 9 10 - 1 - IPE 2203-LecturesMD Al-AminNo ratings yet
- Ph8251 Ms QB - MainDocument48 pagesPh8251 Ms QB - MainVeeria Chandran SNo ratings yet
- Unit-2 AnsDocument25 pagesUnit-2 AnsV.Nagaraju 19O17-M-O5ONo ratings yet
- MM235 - Phase Diagram - SMDocument18 pagesMM235 - Phase Diagram - SMUtkarsh MishraNo ratings yet
- L8 Binary Phase Diagrams PDFDocument78 pagesL8 Binary Phase Diagrams PDFSudeepta MondalNo ratings yet
- Phase Rule (Complete)Document48 pagesPhase Rule (Complete)tenguria samriddhNo ratings yet
- Phase DiagramDocument42 pagesPhase DiagramPalak NaikNo ratings yet
- Phase Rule PDFDocument13 pagesPhase Rule PDFPraveen KumarNo ratings yet
- Phase Rule 1Document62 pagesPhase Rule 1arpitpandey494No ratings yet
- Materials Engineering: Interpretation of Phase DiagramDocument12 pagesMaterials Engineering: Interpretation of Phase DiagramSudipta NathNo ratings yet
- Emm HandoutsDocument58 pagesEmm HandoutsDr.A.Maniram KumarNo ratings yet
- Module 2 - Session 3Document31 pagesModule 2 - Session 3DUE DATENo ratings yet
- Phase 1Document9 pagesPhase 1nprashantshekharNo ratings yet
- MODULE 2-Hari ChemDocument83 pagesMODULE 2-Hari ChemKartik KaushikNo ratings yet
- Phase RuleDocument3 pagesPhase Rulekalyanivishal777No ratings yet
- Phase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingDocument35 pagesPhase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingSiratullah ShahNo ratings yet
- MSM-3 P Phases in Solids ( (Intro & Isomorphous System) PDFDocument15 pagesMSM-3 P Phases in Solids ( (Intro & Isomorphous System) PDFShashank SinghNo ratings yet
- Phase DiagramsDocument50 pagesPhase DiagramsIbrahim MalikNo ratings yet
- Phase Diagrams Study NotesDocument13 pagesPhase Diagrams Study NotesSushilNo ratings yet
- Materials Science - Alloys System - 2Document16 pagesMaterials Science - Alloys System - 2Sumit JoshiNo ratings yet
- Ch8 PhaseDiagramDocument20 pagesCh8 PhaseDiagramThrishnaa BalasupurManiamNo ratings yet
- Part 9 - Phase DiagramsDocument10 pagesPart 9 - Phase DiagramsIsaac YoonNo ratings yet
- 06 Phase DiagramsDocument45 pages06 Phase Diagramskathleen avisoNo ratings yet
- Thermal Equilibrium DiagramDocument65 pagesThermal Equilibrium DiagramShohel RanaNo ratings yet
- Slides - 4Document55 pagesSlides - 4Rahul PandeyNo ratings yet
- Phase 3Document35 pagesPhase 3Faria Sultana MimiNo ratings yet
- Unit 1 MsDocument126 pagesUnit 1 MsHarishNo ratings yet
- Spring End Exam 2013 RevisedDocument12 pagesSpring End Exam 2013 Revisedtanishka narayanNo ratings yet
- Introduction To Engineering Materials: 1 Sanjib Jaypuria, SME, KIIT UniversityDocument21 pagesIntroduction To Engineering Materials: 1 Sanjib Jaypuria, SME, KIIT Universitytanishka narayanNo ratings yet
- Emphasize More On These Topics: Problems ProblemsDocument1 pageEmphasize More On These Topics: Problems Problemstanishka narayanNo ratings yet
- Three Phase Power MeasurementDocument3 pagesThree Phase Power Measurementtanishka narayanNo ratings yet
- BonfiglioliDocument18 pagesBonfiglioliidontlikeebooksNo ratings yet
- PE QuestionBankDocument6 pagesPE QuestionBankRakshith0% (1)
- Alp2 D22 EpDocument1 pageAlp2 D22 EpJefatura de Planta Invemet PeruNo ratings yet
- Premier EX Pro: Combined Fire & Extinguishing Control PanelDocument2 pagesPremier EX Pro: Combined Fire & Extinguishing Control PanelmotaNo ratings yet
- Specification Sheet: Specifications TV2000, TV4000, TV7000DCDocument6 pagesSpecification Sheet: Specifications TV2000, TV4000, TV7000DCAatir AhmedNo ratings yet
- Instruction Bulletin Altivar 61/71 Spare Parts Kits: ® Required Documentation For Use With CD-ROM 8800EP0801 V4.0Document28 pagesInstruction Bulletin Altivar 61/71 Spare Parts Kits: ® Required Documentation For Use With CD-ROM 8800EP0801 V4.0LeandroNo ratings yet
- Ubtd Diesel Rotary Ups Brochure enDocument12 pagesUbtd Diesel Rotary Ups Brochure enhelmy muktiNo ratings yet
- Garnitura SpirometalicaDocument3 pagesGarnitura SpirometalicaRadoo NephilaNo ratings yet
- ForgingDocument56 pagesForgingZulfikarUdenNo ratings yet
- Photodiode Investigatory ProjectDocument25 pagesPhotodiode Investigatory ProjectNishant KumarNo ratings yet
- Petrosjan, M. I - Rock Breakage by Blasting-Balkema (1994) PDFDocument156 pagesPetrosjan, M. I - Rock Breakage by Blasting-Balkema (1994) PDFahmedNo ratings yet
- Physics Lab Exp 1Document4 pagesPhysics Lab Exp 1Shaira EstrellaNo ratings yet
- 0012DI3630 2719 T-SV 1100.001 (En) - Derrick & Risers-Utility PipingDocument9 pages0012DI3630 2719 T-SV 1100.001 (En) - Derrick & Risers-Utility PipingAkhtar AnsariNo ratings yet
- Newton's Law of Motion and Forces: PHY01: General Physics 1 Senior High School DepartmentDocument26 pagesNewton's Law of Motion and Forces: PHY01: General Physics 1 Senior High School DepartmentcookingNo ratings yet
- Experiment 1 Lab ReportDocument21 pagesExperiment 1 Lab ReportOisin MaguireNo ratings yet
- Chapter - 3 (Dynamics)Document10 pagesChapter - 3 (Dynamics)abrarosmanahamedNo ratings yet
- Notebook ScifairDocument40 pagesNotebook Scifairapi-331985309No ratings yet
- A1P6Document6 pagesA1P6leonardo_melo_costaNo ratings yet