100% found this document useful (1 vote)
694 views36 pagesNeural Tube Defects: Dr.M.G.Kartheeka Fellow in Neonatology Cloudnine, OAR
This document provides an overview of neural tube defects (NTDs), including:
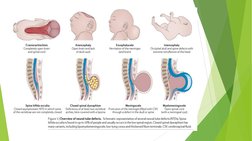
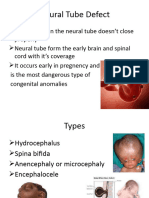
1. NTDs are severe birth defects affecting 1 in 1000 pregnancies and include spina bifida and anencephaly.
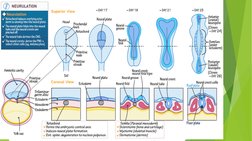
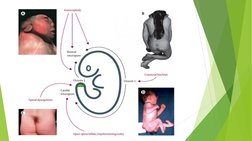
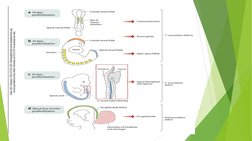
2. Spina bifida, the most common NTD, occurs when the spinal column fails to close properly during development, often resulting in neurological deficits depending on the lesion level.
3. Periconceptional folic acid supplementation has been shown to significantly reduce NTD risk, and food fortification programs have further increased prevention.
4. Prenatal screening and diagnosis involves ultrasound exams and biochemical testing to detect NTDs. Fetal surgery may help in some spina bifida cases
Uploaded by
M G KARTHEEKACopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd
100% found this document useful (1 vote)
694 views36 pagesNeural Tube Defects: Dr.M.G.Kartheeka Fellow in Neonatology Cloudnine, OAR
This document provides an overview of neural tube defects (NTDs), including:
1. NTDs are severe birth defects affecting 1 in 1000 pregnancies and include spina bifida and anencephaly.
2. Spina bifida, the most common NTD, occurs when the spinal column fails to close properly during development, often resulting in neurological deficits depending on the lesion level.
3. Periconceptional folic acid supplementation has been shown to significantly reduce NTD risk, and food fortification programs have further increased prevention.
4. Prenatal screening and diagnosis involves ultrasound exams and biochemical testing to detect NTDs. Fetal surgery may help in some spina bifida cases
Uploaded by
M G KARTHEEKACopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPTX, PDF, TXT or read online on Scribd