Professional Documents
Culture Documents
Annrheumdis 2020 January 79 1 19
Annrheumdis 2020 January 79 1 19
Uploaded by
Ranindya PutriCopyright:
Available Formats
You might also like
- Thionamides: Side Effects and ToxicitiesDocument11 pagesThionamides: Side Effects and ToxicitiesJesús MoraNo ratings yet
- B.SC Ii YearDocument37 pagesB.SC Ii Yearvaishali TMU studentNo ratings yet
- Jcem2157 PDFDocument6 pagesJcem2157 PDFRidwan Putra Beng-BengNo ratings yet
- Methotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Document4 pagesMethotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Saifuddin HaswareNo ratings yet
- CPG Rheumatic ArthritisDocument15 pagesCPG Rheumatic ArthritisCece PaduaNo ratings yet
- DMT and WBC InfectonDocument11 pagesDMT and WBC InfectonRenju KuriakoseNo ratings yet
- RA Management UpdateDocument43 pagesRA Management UpdateFaheem Ul HasanNo ratings yet
- Clinical Case 1Document10 pagesClinical Case 1aveekumbharNo ratings yet
- Full TextDocument9 pagesFull Textbodeadumitru9261No ratings yet
- Rheumatoid Arthritis: Ika Norcahyanti Fakultas Farmasi UNEJDocument59 pagesRheumatoid Arthritis: Ika Norcahyanti Fakultas Farmasi UNEJnimas putriNo ratings yet
- SELECT-MONOTHERAPY FullDocument9 pagesSELECT-MONOTHERAPY FullAlaitz GurreaNo ratings yet
- (19330693 - Journal of Neurosurgery) Riluzole Enhances The Antitumor Effects of Temozolomide Via Suppression of MGMT Expression in GlioblastomaDocument10 pages(19330693 - Journal of Neurosurgery) Riluzole Enhances The Antitumor Effects of Temozolomide Via Suppression of MGMT Expression in GlioblastomaPaul ElidaNo ratings yet
- Drug Therapy in RaDocument37 pagesDrug Therapy in Rasantosh subediNo ratings yet
- AbstractDocument1 pageAbstractkathy briggsNo ratings yet
- Rheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisDocument15 pagesRheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisFadilaNo ratings yet
- DC 180588Document14 pagesDC 180588suryawan0290No ratings yet
- Regular ArticleDocument8 pagesRegular ArticleFaMe TanapornNo ratings yet
- Jurnal 1Document4 pagesJurnal 1dr DevyNo ratings yet
- Vildagliptin Efficacy in Combination With Metformin Among Jordanian Patients With Type 2 Diabetes Mellitus Inadequately Controlled With MetforminDocument5 pagesVildagliptin Efficacy in Combination With Metformin Among Jordanian Patients With Type 2 Diabetes Mellitus Inadequately Controlled With MetforminAlbert EdoNo ratings yet
- Arterita Temp TratmnrtDocument7 pagesArterita Temp TratmnrtCarmen-BadeaNo ratings yet
- Psoriasis Treatments GuidelinesDocument33 pagesPsoriasis Treatments GuidelinesWay FastNo ratings yet
- CA180-274 Synopsis RedactedDocument7 pagesCA180-274 Synopsis RedactedAnonymous jjdtnLrxaNo ratings yet
- A Brief Review of GepantsDocument10 pagesA Brief Review of GepantsFarid Santiago Abedrabbo LombeydaNo ratings yet
- Wang 2018Document42 pagesWang 2018Erni SalehNo ratings yet
- Cannabis Et CancerDocument5 pagesCannabis Et CancerJoaquim MurtinhoNo ratings yet
- Treatment of Glioblastoma With Herbal MedicinesDocument14 pagesTreatment of Glioblastoma With Herbal MedicinesGabrielAbarcaNo ratings yet
- Week 3 BiologicsDocument8 pagesWeek 3 BiologicsShree Narayan YadavNo ratings yet
- 2022 Article 1269Document15 pages2022 Article 1269Virna FigueroaNo ratings yet
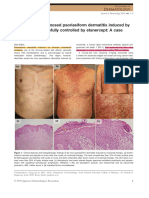
- Histologically-Diagnosed Psoriasiform Dermatitis Induced by Nivolumab Successfully Controlled by Etanercept - A Case ReportDocument2 pagesHistologically-Diagnosed Psoriasiform Dermatitis Induced by Nivolumab Successfully Controlled by Etanercept - A Case ReportM CostantinoNo ratings yet
- The Use of Tocilizumab in Combination With Methotrexate in Indonesian Rheumatoid Arthritis Patients (PICTURE INA Study)Document7 pagesThe Use of Tocilizumab in Combination With Methotrexate in Indonesian Rheumatoid Arthritis Patients (PICTURE INA Study)Intan Winta PratiwiNo ratings yet
- Will Pharmacogenetics Allow Better Prediction of MTX Toxicity and Efficacy in Patients With Rheumatoid ArthritisDocument7 pagesWill Pharmacogenetics Allow Better Prediction of MTX Toxicity and Efficacy in Patients With Rheumatoid Arthritisdoc0814No ratings yet
- Her N Ndez Rodrguez 2009Document12 pagesHer N Ndez Rodrguez 2009Gino Paul Bermeo ValcarcelNo ratings yet
- Treatment of Giant Cell Arteritis (GCA)Document13 pagesTreatment of Giant Cell Arteritis (GCA)Kori Karina Cueva TovarNo ratings yet
- LupusDocument8 pagesLupusFerdinand YuzonNo ratings yet
- Advances in Therapeutic Drug Monitoring For Small-Molecule and Biologic Therapies in Inflammatory Bowel DiseaseDocument19 pagesAdvances in Therapeutic Drug Monitoring For Small-Molecule and Biologic Therapies in Inflammatory Bowel Diseasepaula costa LeandroNo ratings yet
- METFORMINDocument11 pagesMETFORMINsalsabila JacobNo ratings yet
- Tacrolimus and Mycophenolate Mofetil As Second Line Therapies For Pediatric Patients With Autoimmune HepatitisDocument7 pagesTacrolimus and Mycophenolate Mofetil As Second Line Therapies For Pediatric Patients With Autoimmune Hepatitisrhegi isdiara FairuzNo ratings yet
- Tirzepatide Journal ClubDocument4 pagesTirzepatide Journal ClubAhmed KhaledNo ratings yet
- Methotrexate For Rheumatoid Arthritis Patients Who Are On Hemodialysis PDFDocument3 pagesMethotrexate For Rheumatoid Arthritis Patients Who Are On Hemodialysis PDFaporo910No ratings yet
- Journal Round Up Q1 2020Document2 pagesJournal Round Up Q1 2020WillNo ratings yet
- Giant Cell Arteritis and Polymyalgia RheumaticaDocument17 pagesGiant Cell Arteritis and Polymyalgia RheumaticaXochil SolanoNo ratings yet
- PingZhuetal 2017 Tumortreatingfields Anovelandeffectivetherapyforglioblastoma Mechanism Efficacy SafetyandfutureperspectivesDocument15 pagesPingZhuetal 2017 Tumortreatingfields Anovelandeffectivetherapyforglioblastoma Mechanism Efficacy SafetyandfutureperspectivesCf FungNo ratings yet
- Glucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesDocument13 pagesGlucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesSundas EjazNo ratings yet
- Hepatotoxicity During Maintenance Therapy and PrognosisDocument6 pagesHepatotoxicity During Maintenance Therapy and PrognosisRonnyMulyonoNo ratings yet
- Patch Testing and Immunosuppression: A Comprehensive ReviewDocument12 pagesPatch Testing and Immunosuppression: A Comprehensive Reviewnadia apriliaNo ratings yet
- Bortezomib, Lenalidomide, and Dexamethasone As Induction Therapy Prior To Autologous Transplant in Multiple Myeloma PDFDocument9 pagesBortezomib, Lenalidomide, and Dexamethasone As Induction Therapy Prior To Autologous Transplant in Multiple Myeloma PDFivanlchNo ratings yet
- 583Document9 pages583Raichu Si Como NoNo ratings yet
- Gerd R Burmester, William F Rigby, Ronald F Van Vollenhoven, Jonathan Kay, Andrea Rubbert-Roth, Ricardo Blanco, Alysha Kadva, Sophie DimonacoDocument6 pagesGerd R Burmester, William F Rigby, Ronald F Van Vollenhoven, Jonathan Kay, Andrea Rubbert-Roth, Ricardo Blanco, Alysha Kadva, Sophie Dimonaconurul annisaNo ratings yet
- Oncologie GistDocument6 pagesOncologie GistMada IacobNo ratings yet
- Nateglinide Tablets: GeriatricDocument2 pagesNateglinide Tablets: GeriatricUlfa AlyaNo ratings yet
- Rheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisDocument29 pagesRheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisSundas EjazNo ratings yet
- What GPs Need To Know About KetamineDocument4 pagesWhat GPs Need To Know About KetamineAdiAri RosiuNo ratings yet
- SLE ManagementDocument66 pagesSLE ManagementAhnaf MustaviNo ratings yet
- Lenalidomide Plus Prednisone Results in Durable Clinical, Histopathologic, and Molecular Responses in Patients With MyelofibrosisDocument7 pagesLenalidomide Plus Prednisone Results in Durable Clinical, Histopathologic, and Molecular Responses in Patients With MyelofibrosisRima RahmounNo ratings yet
- MRX Clinical Alert-April 2019Document5 pagesMRX Clinical Alert-April 2019Nattawat TeerawattanapongNo ratings yet
- Terapias Combinadas PRRT 2021Document13 pagesTerapias Combinadas PRRT 2021anapinto6388No ratings yet
- Georgianos 2023 Therapeutic Advances in Diabetic KiDocument12 pagesGeorgianos 2023 Therapeutic Advances in Diabetic Kiiacob ancutaNo ratings yet
- J J Rad Oncol 3 2 029-2Document4 pagesJ J Rad Oncol 3 2 029-2Angela PagliusoNo ratings yet
- Abstract Type: No Preference Abstract Submission No.:: WFNOS 2022 Top 10 Session 2 - March 26 (Sat) 10:15-11:30Document1 pageAbstract Type: No Preference Abstract Submission No.:: WFNOS 2022 Top 10 Session 2 - March 26 (Sat) 10:15-11:30martin Ignacio Zapata LaguadoNo ratings yet
- 111A Cobak Latian SoalDocument8 pages111A Cobak Latian SoalRanindya PutriNo ratings yet
- Case Study On Pemphigus VulgarisDocument2 pagesCase Study On Pemphigus VulgarisRanindya PutriNo ratings yet
- 360-Article Text-929-1-10-20210507Document6 pages360-Article Text-929-1-10-20210507Ranindya PutriNo ratings yet
- 2018 Update of The EULAR Recommendations For The Management of Large Vessel VasculitisDocument19 pages2018 Update of The EULAR Recommendations For The Management of Large Vessel VasculitisRanindya PutriNo ratings yet
- Leg UlcerDocument28 pagesLeg UlcerRanindya PutriNo ratings yet
- ANDHITA RESTU DAMAYANTI 22010112130121 Lap - KTI Bab7Document30 pagesANDHITA RESTU DAMAYANTI 22010112130121 Lap - KTI Bab7Ranindya PutriNo ratings yet
Annrheumdis 2020 January 79 1 19
Annrheumdis 2020 January 79 1 19
Uploaded by
Ranindya PutriOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Annrheumdis 2020 January 79 1 19
Annrheumdis 2020 January 79 1 19
Uploaded by
Ranindya PutriCopyright:
Available Formats
The 2018 EULAR algorithm for pharmacological treatment of Takayasu arteritis (TAK).
csDMARD,
conventional synthetic disease modifying anti-rheumatic drug; GC, glucocorticoids; TNF, tumour
necrosis factor; 1A clinical diagnosis of TAK should be confirmed by imaging2; in patients with
more localised disease consider lower initial dose of 25–30 mg/day3; methotrexate or
mycophenolate mofetil, leflunomide or azathioprine (when a patient does not tolerate the first
choice drug switching is an option) or cyclophosphamide (only if other treatments have failed or
have not been tolerated) can be used as an alternative4; or 5–15 mg above the last effective
dose5; see table 2 for definitions6; the treatment target is sustained remission (absence of
clinical signs and symptoms of active Tak associated with normal acute phase reactants) plus
ability to taper GCs to the specified target without relapse7; tocilzumab is not formally licensed
for use in TAK in the European Union (EU), the recommended dose is 162 mg one time per week
s.c.8; Tumor necrosis factor (TNF)-inhibitors are not formally licensed for use in TAK (when a
patient does not tolerate the first choice biological switching is an option).
Bernhard Hellmich et al. Ann Rheum Dis 2020;79:19-30
©2020 by BMJ Publishing Group Ltd and European League Against Rheumatism
You might also like
- Thionamides: Side Effects and ToxicitiesDocument11 pagesThionamides: Side Effects and ToxicitiesJesús MoraNo ratings yet
- B.SC Ii YearDocument37 pagesB.SC Ii Yearvaishali TMU studentNo ratings yet
- Jcem2157 PDFDocument6 pagesJcem2157 PDFRidwan Putra Beng-BengNo ratings yet
- Methotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Document4 pagesMethotrexate in Rheumatoid Arthritis Efficacy and Safety 2329 6887 2 127Saifuddin HaswareNo ratings yet
- CPG Rheumatic ArthritisDocument15 pagesCPG Rheumatic ArthritisCece PaduaNo ratings yet
- DMT and WBC InfectonDocument11 pagesDMT and WBC InfectonRenju KuriakoseNo ratings yet
- RA Management UpdateDocument43 pagesRA Management UpdateFaheem Ul HasanNo ratings yet
- Clinical Case 1Document10 pagesClinical Case 1aveekumbharNo ratings yet
- Full TextDocument9 pagesFull Textbodeadumitru9261No ratings yet
- Rheumatoid Arthritis: Ika Norcahyanti Fakultas Farmasi UNEJDocument59 pagesRheumatoid Arthritis: Ika Norcahyanti Fakultas Farmasi UNEJnimas putriNo ratings yet
- SELECT-MONOTHERAPY FullDocument9 pagesSELECT-MONOTHERAPY FullAlaitz GurreaNo ratings yet
- (19330693 - Journal of Neurosurgery) Riluzole Enhances The Antitumor Effects of Temozolomide Via Suppression of MGMT Expression in GlioblastomaDocument10 pages(19330693 - Journal of Neurosurgery) Riluzole Enhances The Antitumor Effects of Temozolomide Via Suppression of MGMT Expression in GlioblastomaPaul ElidaNo ratings yet
- Drug Therapy in RaDocument37 pagesDrug Therapy in Rasantosh subediNo ratings yet
- AbstractDocument1 pageAbstractkathy briggsNo ratings yet
- Rheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisDocument15 pagesRheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisFadilaNo ratings yet
- DC 180588Document14 pagesDC 180588suryawan0290No ratings yet
- Regular ArticleDocument8 pagesRegular ArticleFaMe TanapornNo ratings yet
- Jurnal 1Document4 pagesJurnal 1dr DevyNo ratings yet
- Vildagliptin Efficacy in Combination With Metformin Among Jordanian Patients With Type 2 Diabetes Mellitus Inadequately Controlled With MetforminDocument5 pagesVildagliptin Efficacy in Combination With Metformin Among Jordanian Patients With Type 2 Diabetes Mellitus Inadequately Controlled With MetforminAlbert EdoNo ratings yet
- Arterita Temp TratmnrtDocument7 pagesArterita Temp TratmnrtCarmen-BadeaNo ratings yet
- Psoriasis Treatments GuidelinesDocument33 pagesPsoriasis Treatments GuidelinesWay FastNo ratings yet
- CA180-274 Synopsis RedactedDocument7 pagesCA180-274 Synopsis RedactedAnonymous jjdtnLrxaNo ratings yet
- A Brief Review of GepantsDocument10 pagesA Brief Review of GepantsFarid Santiago Abedrabbo LombeydaNo ratings yet
- Wang 2018Document42 pagesWang 2018Erni SalehNo ratings yet
- Cannabis Et CancerDocument5 pagesCannabis Et CancerJoaquim MurtinhoNo ratings yet
- Treatment of Glioblastoma With Herbal MedicinesDocument14 pagesTreatment of Glioblastoma With Herbal MedicinesGabrielAbarcaNo ratings yet
- Week 3 BiologicsDocument8 pagesWeek 3 BiologicsShree Narayan YadavNo ratings yet
- 2022 Article 1269Document15 pages2022 Article 1269Virna FigueroaNo ratings yet
- Histologically-Diagnosed Psoriasiform Dermatitis Induced by Nivolumab Successfully Controlled by Etanercept - A Case ReportDocument2 pagesHistologically-Diagnosed Psoriasiform Dermatitis Induced by Nivolumab Successfully Controlled by Etanercept - A Case ReportM CostantinoNo ratings yet
- The Use of Tocilizumab in Combination With Methotrexate in Indonesian Rheumatoid Arthritis Patients (PICTURE INA Study)Document7 pagesThe Use of Tocilizumab in Combination With Methotrexate in Indonesian Rheumatoid Arthritis Patients (PICTURE INA Study)Intan Winta PratiwiNo ratings yet
- Will Pharmacogenetics Allow Better Prediction of MTX Toxicity and Efficacy in Patients With Rheumatoid ArthritisDocument7 pagesWill Pharmacogenetics Allow Better Prediction of MTX Toxicity and Efficacy in Patients With Rheumatoid Arthritisdoc0814No ratings yet
- Her N Ndez Rodrguez 2009Document12 pagesHer N Ndez Rodrguez 2009Gino Paul Bermeo ValcarcelNo ratings yet
- Treatment of Giant Cell Arteritis (GCA)Document13 pagesTreatment of Giant Cell Arteritis (GCA)Kori Karina Cueva TovarNo ratings yet
- LupusDocument8 pagesLupusFerdinand YuzonNo ratings yet
- Advances in Therapeutic Drug Monitoring For Small-Molecule and Biologic Therapies in Inflammatory Bowel DiseaseDocument19 pagesAdvances in Therapeutic Drug Monitoring For Small-Molecule and Biologic Therapies in Inflammatory Bowel Diseasepaula costa LeandroNo ratings yet
- METFORMINDocument11 pagesMETFORMINsalsabila JacobNo ratings yet
- Tacrolimus and Mycophenolate Mofetil As Second Line Therapies For Pediatric Patients With Autoimmune HepatitisDocument7 pagesTacrolimus and Mycophenolate Mofetil As Second Line Therapies For Pediatric Patients With Autoimmune Hepatitisrhegi isdiara FairuzNo ratings yet
- Tirzepatide Journal ClubDocument4 pagesTirzepatide Journal ClubAhmed KhaledNo ratings yet
- Methotrexate For Rheumatoid Arthritis Patients Who Are On Hemodialysis PDFDocument3 pagesMethotrexate For Rheumatoid Arthritis Patients Who Are On Hemodialysis PDFaporo910No ratings yet
- Journal Round Up Q1 2020Document2 pagesJournal Round Up Q1 2020WillNo ratings yet
- Giant Cell Arteritis and Polymyalgia RheumaticaDocument17 pagesGiant Cell Arteritis and Polymyalgia RheumaticaXochil SolanoNo ratings yet
- PingZhuetal 2017 Tumortreatingfields Anovelandeffectivetherapyforglioblastoma Mechanism Efficacy SafetyandfutureperspectivesDocument15 pagesPingZhuetal 2017 Tumortreatingfields Anovelandeffectivetherapyforglioblastoma Mechanism Efficacy SafetyandfutureperspectivesCf FungNo ratings yet
- Glucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesDocument13 pagesGlucocorticoids in Systemic Lupus Erythematosus. Ten Questions and Some IssuesSundas EjazNo ratings yet
- Hepatotoxicity During Maintenance Therapy and PrognosisDocument6 pagesHepatotoxicity During Maintenance Therapy and PrognosisRonnyMulyonoNo ratings yet
- Patch Testing and Immunosuppression: A Comprehensive ReviewDocument12 pagesPatch Testing and Immunosuppression: A Comprehensive Reviewnadia apriliaNo ratings yet
- Bortezomib, Lenalidomide, and Dexamethasone As Induction Therapy Prior To Autologous Transplant in Multiple Myeloma PDFDocument9 pagesBortezomib, Lenalidomide, and Dexamethasone As Induction Therapy Prior To Autologous Transplant in Multiple Myeloma PDFivanlchNo ratings yet
- 583Document9 pages583Raichu Si Como NoNo ratings yet
- Gerd R Burmester, William F Rigby, Ronald F Van Vollenhoven, Jonathan Kay, Andrea Rubbert-Roth, Ricardo Blanco, Alysha Kadva, Sophie DimonacoDocument6 pagesGerd R Burmester, William F Rigby, Ronald F Van Vollenhoven, Jonathan Kay, Andrea Rubbert-Roth, Ricardo Blanco, Alysha Kadva, Sophie Dimonaconurul annisaNo ratings yet
- Oncologie GistDocument6 pagesOncologie GistMada IacobNo ratings yet
- Nateglinide Tablets: GeriatricDocument2 pagesNateglinide Tablets: GeriatricUlfa AlyaNo ratings yet
- Rheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisDocument29 pagesRheumatoid Arthritis Drug Treatment For Rheumatoid ArthritisSundas EjazNo ratings yet
- What GPs Need To Know About KetamineDocument4 pagesWhat GPs Need To Know About KetamineAdiAri RosiuNo ratings yet
- SLE ManagementDocument66 pagesSLE ManagementAhnaf MustaviNo ratings yet
- Lenalidomide Plus Prednisone Results in Durable Clinical, Histopathologic, and Molecular Responses in Patients With MyelofibrosisDocument7 pagesLenalidomide Plus Prednisone Results in Durable Clinical, Histopathologic, and Molecular Responses in Patients With MyelofibrosisRima RahmounNo ratings yet
- MRX Clinical Alert-April 2019Document5 pagesMRX Clinical Alert-April 2019Nattawat TeerawattanapongNo ratings yet
- Terapias Combinadas PRRT 2021Document13 pagesTerapias Combinadas PRRT 2021anapinto6388No ratings yet
- Georgianos 2023 Therapeutic Advances in Diabetic KiDocument12 pagesGeorgianos 2023 Therapeutic Advances in Diabetic Kiiacob ancutaNo ratings yet
- J J Rad Oncol 3 2 029-2Document4 pagesJ J Rad Oncol 3 2 029-2Angela PagliusoNo ratings yet
- Abstract Type: No Preference Abstract Submission No.:: WFNOS 2022 Top 10 Session 2 - March 26 (Sat) 10:15-11:30Document1 pageAbstract Type: No Preference Abstract Submission No.:: WFNOS 2022 Top 10 Session 2 - March 26 (Sat) 10:15-11:30martin Ignacio Zapata LaguadoNo ratings yet
- 111A Cobak Latian SoalDocument8 pages111A Cobak Latian SoalRanindya PutriNo ratings yet
- Case Study On Pemphigus VulgarisDocument2 pagesCase Study On Pemphigus VulgarisRanindya PutriNo ratings yet
- 360-Article Text-929-1-10-20210507Document6 pages360-Article Text-929-1-10-20210507Ranindya PutriNo ratings yet
- 2018 Update of The EULAR Recommendations For The Management of Large Vessel VasculitisDocument19 pages2018 Update of The EULAR Recommendations For The Management of Large Vessel VasculitisRanindya PutriNo ratings yet
- Leg UlcerDocument28 pagesLeg UlcerRanindya PutriNo ratings yet
- ANDHITA RESTU DAMAYANTI 22010112130121 Lap - KTI Bab7Document30 pagesANDHITA RESTU DAMAYANTI 22010112130121 Lap - KTI Bab7Ranindya PutriNo ratings yet