Professional Documents
Culture Documents
1.3a Intro To Hydrolysis and Dehydration Synthesis
1.3a Intro To Hydrolysis and Dehydration Synthesis
Uploaded by
Soleil R.0 ratings0% found this document useful (0 votes)
25 views4 pagesThis document introduces important biochemical reactions including hydrolysis and dehydration synthesis. Hydrolysis is a catabolic reaction that breaks polymers such as carbohydrates, lipids, proteins, and nucleic acids into monomers by adding a water molecule. Dehydration synthesis is an anabolic reaction that forms polymers from monomers by removing a water molecule, requiring energy. These reactions of hydrolysis and dehydration synthesis are used to break down and build up the four major macromolecules in living organisms.
Original Description:
Original Title
1.3a Intro to Hydrolysis and Dehydration Synthesis
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document introduces important biochemical reactions including hydrolysis and dehydration synthesis. Hydrolysis is a catabolic reaction that breaks polymers such as carbohydrates, lipids, proteins, and nucleic acids into monomers by adding a water molecule. Dehydration synthesis is an anabolic reaction that forms polymers from monomers by removing a water molecule, requiring energy. These reactions of hydrolysis and dehydration synthesis are used to break down and build up the four major macromolecules in living organisms.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
25 views4 pages1.3a Intro To Hydrolysis and Dehydration Synthesis
1.3a Intro To Hydrolysis and Dehydration Synthesis
Uploaded by
Soleil R.This document introduces important biochemical reactions including hydrolysis and dehydration synthesis. Hydrolysis is a catabolic reaction that breaks polymers such as carbohydrates, lipids, proteins, and nucleic acids into monomers by adding a water molecule. Dehydration synthesis is an anabolic reaction that forms polymers from monomers by removing a water molecule, requiring energy. These reactions of hydrolysis and dehydration synthesis are used to break down and build up the four major macromolecules in living organisms.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 4
Introduction to Biochemical Reactions:
Hydrolysis and Dehydration Synthesis
Important terms:
•Monomer- single molecule or
“building block” that can form
polymers
•Polymer- long/large molecule of
repeating monomers
•Polymerization- reactions forming a
polymer
Important Terms:
All chemical reactions within a living organism can be classified
as:
•Anabolic reaction- chemical reactions in which simpler
substances are combined to form more complex molecules.
Anabolic reactions usually require energy. Anabolic reactions build
new molecules and/or store energy.
•Catabolic reaction- chemical reactions that result in the
breakdown of more complex organic molecules into simpler
substances. Release energy that is stored (in ATP) or used to drive
anabolic reactions.
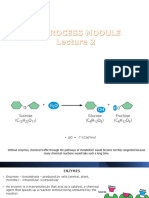
Hydrolysis (polymer->monomers)
• A chemical reaction that results in cleavage of a covalent bond with the
addition of a water molecule -(Hydro = water; lysis = break);
• a reaction process that breaks covalent bonds between monomers by the
addition of water molecules.
• A hydrogen from the water bonds to one monomer and the hydroxyl
bonds to the adjacent monomer. The covalent bond between these
monomers breaks and the larger molecule (polymer) is split into smaller
molecules (monomers)
• Releases energy – “catabolic”
Dehydration Synthesis (monomers-> polymer
“polymerization”)
*Note: your textbook describes Dehydration Synthesis as “Condensation”
•A chemical reaction that results in the formation of a covalent bond between
two molecules (“synthesis”) by removing a water molecule (“dehydration”)
•One monomer loses a hydroxyl (–OH), and the other monomer loses a
hydrogen (–H). (the H usually comes from a functional group)
•requires energy – “anabolic” reaction
•requires biological catalysts or enzymes.
Each of the four macromolecules (Carbohydrates, Lipids, Proteins and
Nucleic Acids) use dehydration synthesis reactions to build polymers and
hydrolysis reactions to break polymers into monomers.
You might also like
- BiochemistryDocument6 pagesBiochemistryBlaine Rogalski100% (14)
- 2 the+Chemistry+of+LifeDocument107 pages2 the+Chemistry+of+Lifegabbs_123No ratings yet
- Quantitative Human Physiology: An IntroductionFrom EverandQuantitative Human Physiology: An IntroductionRating: 2 out of 5 stars2/5 (1)
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Foundations in Microbiology: Microbial Metabolism: The Chemical Crossroads of Life TalaroDocument48 pagesFoundations in Microbiology: Microbial Metabolism: The Chemical Crossroads of Life TalaroDennis Nabor Muñoz, RN,RMNo ratings yet
- BT1000 - Cellular Metabolism PDFDocument35 pagesBT1000 - Cellular Metabolism PDFAnubhavAgarwal100% (1)
- BIOCHEMISTRY 106 Lecture RevisedDocument46 pagesBIOCHEMISTRY 106 Lecture Revisedjoanbless79No ratings yet
- Introduction To Chemistry of LifeDocument27 pagesIntroduction To Chemistry of LifeJezterE.BalladosItsujiNo ratings yet
- Water: The Solvent of Life Where There Is Water, There Is LifeDocument18 pagesWater: The Solvent of Life Where There Is Water, There Is LifeIsaiah Fidelis Maji100% (1)
- 3.1: Synthesis of Biological MacromoleculesDocument2 pages3.1: Synthesis of Biological MacromoleculesElah TapangNo ratings yet
- BIOL 1103 Foundations of Biology I: Unit 1 - Module 2: Life's Molecules Pt.iDocument7 pagesBIOL 1103 Foundations of Biology I: Unit 1 - Module 2: Life's Molecules Pt.iShalina MalikNo ratings yet
- Unit 2 Molecules - 281123Document37 pagesUnit 2 Molecules - 281123DAN DNANo ratings yet
- Introduction To Nutritional BiochemistryDocument20 pagesIntroduction To Nutritional BiochemistryKhaledNo ratings yet
- Lecture 1 - Introduction To Nutritional Biochemistry - TaggedDocument20 pagesLecture 1 - Introduction To Nutritional Biochemistry - Taggedhashm.f.alamerNo ratings yet
- ABS 311 Cell Biology: The World of The Cell by Becker, Kleinsmith, Hardin 8 Edition Chapter 2: The Chemistry of The CellDocument33 pagesABS 311 Cell Biology: The World of The Cell by Becker, Kleinsmith, Hardin 8 Edition Chapter 2: The Chemistry of The CellKelsey WhitmoreNo ratings yet
- Lec (Master) - Chemistry 2Document55 pagesLec (Master) - Chemistry 2Swapnil PandyaNo ratings yet
- Chapter 4-IDocument40 pagesChapter 4-ISifan MotumaNo ratings yet
- Metabolism (Compatibility Mode)Document13 pagesMetabolism (Compatibility Mode)Dark_KiroNo ratings yet
- Water & PHDocument42 pagesWater & PHBea SamonteNo ratings yet
- Chapter 3 Reviewer in BiosciDocument8 pagesChapter 3 Reviewer in BiosciAngelica PaulinoNo ratings yet
- cHEMICAL RECATIONSDocument2 pagescHEMICAL RECATIONSAnam FNo ratings yet
- Week 2 Lesson 2Document18 pagesWeek 2 Lesson 2Yury DesuNo ratings yet
- Overview of General and Organic Chemistry and Introduction To BiochemistryDocument47 pagesOverview of General and Organic Chemistry and Introduction To BiochemistrymalvincayabyabNo ratings yet
- NATS 1675 - 3. Chemistry of Life - MacromoleculesDocument9 pagesNATS 1675 - 3. Chemistry of Life - MacromoleculesAmna Zafar GazaNo ratings yet
- Lec 2Document31 pagesLec 2Ramy El-HadadNo ratings yet
- Microbiology Lec 1Document16 pagesMicrobiology Lec 1Mushtaq HassanNo ratings yet
- Lecture BIOCHEMISTRY of CYTOSOL AlfiDocument56 pagesLecture BIOCHEMISTRY of CYTOSOL Alfisennaavia12No ratings yet
- BiologyDocument387 pagesBiologyAaron Wong100% (1)
- 1b - Intro To Macromolecules COMPLETEDocument2 pages1b - Intro To Macromolecules COMPLETEAaliyah AdenNo ratings yet
- PolymersDocument33 pagesPolymersNithishNo ratings yet
- 2.1-2.6 Biology Review 1Document21 pages2.1-2.6 Biology Review 1Victoria FinolNo ratings yet
- Chapter 02 LectureDocument41 pagesChapter 02 Lectureplayer19No ratings yet
- 2.1 2.2 Metabolism+and+Water 2021 Science 10 IBDocument20 pages2.1 2.2 Metabolism+and+Water 2021 Science 10 IBNexExplosiveNo ratings yet
- Bio Notes Test PrepDocument13 pagesBio Notes Test PrepPilot727No ratings yet
- Foundations in Microbiology: TalaroDocument48 pagesFoundations in Microbiology: Talaromertx013No ratings yet
- Lecture 2Document18 pagesLecture 2warden tambiNo ratings yet
- Introduction - Inorganic Chemistry: Biology 2121Document20 pagesIntroduction - Inorganic Chemistry: Biology 2121shyamNo ratings yet
- BioChem Study GuideDocument80 pagesBioChem Study GuidecerscakingNo ratings yet
- Unit Energy Releasing Pathways: StructureDocument33 pagesUnit Energy Releasing Pathways: StructureAD-MQNo ratings yet
- Chem 153A UCLADocument83 pagesChem 153A UCLAChrisOrtNo ratings yet
- Cap 2Document19 pagesCap 2Francesca PrevitaliNo ratings yet
- Lecture 7 Continuation (Biological Molecules)Document13 pagesLecture 7 Continuation (Biological Molecules)Shadreck CharlesNo ratings yet
- The Molecular Logic of Life: Biochemistry Describes in Molecular Terms The Structure, Mechanisms and ChemicalDocument7 pagesThe Molecular Logic of Life: Biochemistry Describes in Molecular Terms The Structure, Mechanisms and ChemicalBakhita MaryamNo ratings yet
- BMSCDocument40 pagesBMSCliz.murphy2004No ratings yet
- BiochemistryDocument5 pagesBiochemistrynaruto710@wuNo ratings yet
- 3.1 Synthesis of Biological Macromolecules Biology 2e OpenStaxDocument2 pages3.1 Synthesis of Biological Macromolecules Biology 2e OpenStaxAlodia PastorizoNo ratings yet
- 6 Introduction of MetabolismDocument22 pages6 Introduction of MetabolismserficasoNo ratings yet
- Chemical Properties of A Carbon AtomdsdddddddddddDocument3 pagesChemical Properties of A Carbon AtomdsdddddddddddMohammed EbrahimNo ratings yet
- Chapter 5 - Microbial MetabolismDocument7 pagesChapter 5 - Microbial MetabolismMelissa G WilliamsNo ratings yet
- L02 Cell Chemistry and BioenergeticsDocument66 pagesL02 Cell Chemistry and BioenergeticsMa Christina Alessandra HingcoNo ratings yet
- Unit 1.2Document4 pagesUnit 1.2ch.town321No ratings yet
- GlossaryChapter1 BiochemDocument5 pagesGlossaryChapter1 BiochemmeretithNo ratings yet
- MMMMMMMMMMDocument7 pagesMMMMMMMMMMfarah.3m2rNo ratings yet
- 2.2 Energy: Chapter 2: Chemistry of LifeDocument13 pages2.2 Energy: Chapter 2: Chemistry of Lifeapi-520057338No ratings yet
- Nature of MetabolismDocument12 pagesNature of MetabolismIshanSaneNo ratings yet
- Topic 1 LectureDocument36 pagesTopic 1 Lecturebibi.hansmuddyNo ratings yet
- MBC 201 Nature of Biomolecules, Basic Concept of Metabolism, Integration of Intermediary Metabolism and Energy Generation.Document13 pagesMBC 201 Nature of Biomolecules, Basic Concept of Metabolism, Integration of Intermediary Metabolism and Energy Generation.Aina AdesolaNo ratings yet
- Onaylanmamış 704688Document39 pagesOnaylanmamış 704688enesNo ratings yet
- Walang MakakaseethisDocument3 pagesWalang MakakaseethisAlissa YangNo ratings yet