Professional Documents
Culture Documents
Chemical Effect of Electric Current
Uploaded by
Sarveshya0 ratings0% found this document useful (0 votes)
12 views10 pagesCopyright
© © All Rights Reserved
Available Formats
PPT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views10 pagesChemical Effect of Electric Current
Uploaded by
SarveshyaCopyright:
© All Rights Reserved
Available Formats
Download as PPT, PDF, TXT or read online from Scribd
You are on page 1of 10
CHAPTER - 14
CHEMICAL EFFECTS OF ELECTRIC
CURRENT
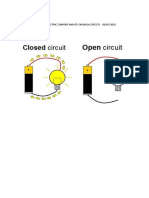
1) Conduction tester :-
A conduction tester is a device used to determine whether a
substance is a good or poor conductor of electricity.
a) Conduction tester using a torch bulb :-
A simple conduction tester has an electric cell and a torch bulb. One
terminal of the cell is connected to one terminal of the bulb by a wire.
The other terminal of the cell and bulb have wires which can be brought
in contact with materials to test whether they are good or poor
conductors of electricity.
If the material is a good conductor, the bulb glows and if it is a poor
conductor the bulb does not glow.
b) Conduction tester using LED :-
Sometimes the current flowing through the circuit may
be too weak and the filament of the bulb may not get
sufficiently heated to make it glow. Then instead of a torch
bulb an LED can be used in the circuit. LED glows even
when a weak current flows in the circuit.
c) Conduction tester using a magnetic compass :-
If a magnetic compass is kept near a wire and current
flows through the wire, the magnetic needle gets deflected.
So we can use a magnetic compass instead of a torch bulb
or LED in the circuit. The magnetic needle gets deflected
even if a weak current flows in the circuit.
2) Electrical conductivity of solids :-
Some solids are good conductors of electricity.
Eg :- copper, steel, iron, aluminium etc.
Some solids are poor conductors of electricity.
Eg :- wood, plastic, rubber, glass etc.
3) Electrical conductivity of liquids :-
Some liquids are good conductors of electricity.
Eg :- tap water, salt solution, hydrochloric acid, sodium hydroxide etc.
(solutions of acids, bases and salts are conductors of electricity)
Some liquids are poor conductor of electricity .
Eg :- distilled water, sugar solution, kerosene, spirit etc.
4) Chemical effects of electric current :-
When electric current passes through a conducting solution, it
causes chemical reactions. This is called chemical effect of electric
current.
Chemical effect of electric current may cause :-
i) Formation of gas bubbles at the electrodes.
ii) Deposit of metal on the electrodes.
iii) Change in colour of the solution.
5) Electrolysis of water :-
When electric current is passed through water, it splits up into
hydrogen and oxygen. This is called electrolysis of water.
When electric current is passed through water oxygen gas bubbles
are produced at the electrode connected to the positive terminal of the
battery and hydrogen gas bubbles are produce at the electrode
connected to the negative terminal of the battery.
6) Electroplating :-
The process of depositing a layer of any desired metal on another
material by means of electricity is called electroplating.
Eg:- For electroplating of copper on an object, the object is dipped in
acidified copper sulphate solution and connected to the negative
terminal of a battery. A copper strip is dipped in the solution and
connected to the positive terminal of the battery. When electric current
is passed through the solution, copper from the copper strip is
deposited on the object.
7) Uses of electroplating :-
Electroplating is used in industry for coating metal objects with a thin
layer of a desired metal.
Chromium has a shiny appearance and it does not corrode. Chromium
plating is done on objects like bicycle parts, car parts, taps, gas
burners, wheel rims etc.
Jewellery makers electroplate gold and silver on less expensive
metals to give an appearance of gold or silver.
Tin cans used for storing food are electroplated with tin over iron
because tin is less reactive than iron and protects iron from corrosion.
Iron objects are coated with zinc to protect it from corrosion.
You might also like
- Chemical EffectsDocument10 pagesChemical Effectsmokshithakapoor657No ratings yet
- Chapter - 14: Chemical Effects of Electric CurrentDocument10 pagesChapter - 14: Chemical Effects of Electric CurrentSriram GurusamyNo ratings yet
- VIII-14-Chemical Effects of Electric CurrentDocument10 pagesVIII-14-Chemical Effects of Electric CurrentBalaji ShanmugamNo ratings yet
- Chemical Effects of Electric CurrentDocument4 pagesChemical Effects of Electric CurrentJayden DcostaNo ratings yet
- QA-Chemical Effects of Electric CurrentDocument6 pagesQA-Chemical Effects of Electric CurrentNikita RajNo ratings yet
- 8 Chemical Effects of Electric CurrentDocument5 pages8 Chemical Effects of Electric Currentandrew andrewNo ratings yet
- Chemical Effect of Current - 8thDocument14 pagesChemical Effect of Current - 8thVijay KumarNo ratings yet
- Chemical Effects 8Document2 pagesChemical Effects 8indrapreet singhNo ratings yet
- ElectroplatingDocument5 pagesElectroplatingPraneet PokhriyalNo ratings yet
- Electricity and Chemical Effects of CurrentDocument3 pagesElectricity and Chemical Effects of CurrentGururaj KulkarniNo ratings yet
- 8th Chemical Effects of Electric Current Solved QuestionsDocument3 pages8th Chemical Effects of Electric Current Solved QuestionsKavitha KNo ratings yet
- Physics Chemical Effects of Electric Current Short Type Questions With Their AnswersDocument3 pagesPhysics Chemical Effects of Electric Current Short Type Questions With Their AnswersUzma MajeedNo ratings yet
- VI 12 Electricity and CircuitsDocument11 pagesVI 12 Electricity and CircuitsAnnAmmusNo ratings yet
- Chemical Effect of Electric Current-5 (2021-22)Document24 pagesChemical Effect of Electric Current-5 (2021-22)Avyam SharmaNo ratings yet
- 2.5 Effect of An Electric Current On SubstancesDocument7 pages2.5 Effect of An Electric Current On Substancesgabrielsuva6No ratings yet
- Electroplating ProcessDocument11 pagesElectroplating ProcessMuhammad AzhariNo ratings yet
- (8th) Chemical Effects of Electric Current Solved AssignmentsDocument3 pages(8th) Chemical Effects of Electric Current Solved AssignmentssushantNo ratings yet
- Revision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentDocument2 pagesRevision Notes Class 8 Science Chapter 14 - Chemical Effects of Electric CurrentArpit SharmaNo ratings yet
- Class 8-Science_ Part 2Document9 pagesClass 8-Science_ Part 2Arnav MalasiNo ratings yet
- 2901242031047882Document7 pages2901242031047882Tvisha SolankiNo ratings yet
- C8 Chemical EffectDocument10 pagesC8 Chemical EffectorukathaisollungakalaijaiNo ratings yet
- Chemical Effects of Electric Current - 1704269942Document12 pagesChemical Effects of Electric Current - 1704269942infoinyourfingertipsNo ratings yet
- Chemical Effect and Electric CurrentDocument3 pagesChemical Effect and Electric CurrentRANJIT KUMAR SHAHNo ratings yet
- Quickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentDocument5 pagesQuickcard Class-VIII (Science) Chapter-14 Chemical Effects of Electric CurrentTridha DuttaNo ratings yet
- Chemical Effects of Electric CurrentDocument9 pagesChemical Effects of Electric CurrentPrabal SinghNo ratings yet
- L_14_Chemical_Effects_of_Electric_CurrentDocument31 pagesL_14_Chemical_Effects_of_Electric_CurrentSARANYA RAWATNo ratings yet
- Electric Current TestingDocument12 pagesElectric Current Testingstory manNo ratings yet
- Chemical Effects of Electric Current-Key NotesDocument6 pagesChemical Effects of Electric Current-Key Notestejasgoel2910No ratings yet
- Basic Electrical Engineering | Er.Menuka DevkotaDocument8 pagesBasic Electrical Engineering | Er.Menuka DevkotaDIKE THAPANo ratings yet
- Chemical Effects of Current: Conductors, Insulators & ElectroplatingDocument10 pagesChemical Effects of Current: Conductors, Insulators & ElectroplatingSifat MongaNo ratings yet
- Experiment-3: Galvanic SeriesDocument4 pagesExperiment-3: Galvanic SeriesChayon MondalNo ratings yet
- CIE Chemistry IGCSE Topic 5 FlashcardsDocument65 pagesCIE Chemistry IGCSE Topic 5 FlashcardsBhawana SinghNo ratings yet
- Chemical EffectDocument12 pagesChemical EffectMohan LalNo ratings yet
- Chemical Effects of Electric CurrentDocument4 pagesChemical Effects of Electric CurrentTanishq SharmaNo ratings yet
- Amta5 6 Applying Shielded Metal Arc Welding Smaw TechniquesDocument132 pagesAmta5 6 Applying Shielded Metal Arc Welding Smaw TechniquesSaurav Kumaar GuptaNo ratings yet
- Electrolysis Grade 9 Science LessonDocument6 pagesElectrolysis Grade 9 Science LessonSwarnapaliliyanageNo ratings yet
- Batteries and Alternative Sources of EnergyDocument33 pagesBatteries and Alternative Sources of EnergyLasana A. TulayNo ratings yet
- Relationship Between Standard Electrode Potential & Rate of ElectrolysisDocument3 pagesRelationship Between Standard Electrode Potential & Rate of ElectrolysisAbbey HeNo ratings yet
- Effects of Electric Current on Chemical SolutionsDocument8 pagesEffects of Electric Current on Chemical SolutionsAbhiNo ratings yet
- Chemical Effets of El NVS TeachersDocument12 pagesChemical Effets of El NVS Teachersaashna shirbhateNo ratings yet
- Dr. N. Srikantamurthy, Dept. of Chemistry Metal FinishingDocument6 pagesDr. N. Srikantamurthy, Dept. of Chemistry Metal FinishingLolNo ratings yet
- * وا ةءافك رثكلاا ةدام وه دوثاكلا اطاشن رثكلاا ةداملا وا ةلكاتملا ةداملا * ةيبطقلا بلق ضرغل يه ةيراطبلا Active to passive * ساحن و ةضفلاو بهذلا لثم دونلااDocument11 pages* وا ةءافك رثكلاا ةدام وه دوثاكلا اطاشن رثكلاا ةداملا وا ةلكاتملا ةداملا * ةيبطقلا بلق ضرغل يه ةيراطبلا Active to passive * ساحن و ةضفلاو بهذلا لثم دونلااMohammed HusseinNo ratings yet
- Chapter 6.2 Redox and ElectrolysisDocument22 pagesChapter 6.2 Redox and ElectrolysisdawsontangxyNo ratings yet
- Chem For Eng 1Document123 pagesChem For Eng 1Am AsdfghjklNo ratings yet
- Chemical Effects of Electric CurrentDocument4 pagesChemical Effects of Electric CurrentÁbìńàv GNo ratings yet
- Electroplating and Corrosion: Unit-4Document50 pagesElectroplating and Corrosion: Unit-4Hadis SyoumNo ratings yet
- 1952 VEDANT - Exp.9.electropl.Document3 pages1952 VEDANT - Exp.9.electropl.Shriansh KulkarniNo ratings yet
- Class-8 ElectricityDocument4 pagesClass-8 ElectricityMamta JoshiNo ratings yet
- Electroplating Project ReportDocument19 pagesElectroplating Project ReportKaran SatiNo ratings yet
- Ch-14 Electric Current and Its EffectsDocument5 pagesCh-14 Electric Current and Its Effectstanshikha dharNo ratings yet
- Electrolysis PDFDocument13 pagesElectrolysis PDFShaikh Irad100% (1)
- Rahul Metal Detector Full ReportDocument23 pagesRahul Metal Detector Full ReportVishal Singh56% (9)
- Chem For Eng 1Document122 pagesChem For Eng 1Lindsay LabagnoyNo ratings yet
- Electroplating and Current Efficiency - UeeeDocument27 pagesElectroplating and Current Efficiency - UeeeTanishq SharmaNo ratings yet
- Study Material - Chemical - Effect - of - Electric Current - classVIIIDocument15 pagesStudy Material - Chemical - Effect - of - Electric Current - classVIIIRajarshiNo ratings yet
- Chemical Effect and Current FoundationDocument16 pagesChemical Effect and Current FoundationAshish SundaNo ratings yet
- 04 Electric Circuits - Effects of An Electric Current 2016Document16 pages04 Electric Circuits - Effects of An Electric Current 2016api-240330821No ratings yet
- Chemical Effects of CurrentDocument7 pagesChemical Effects of CurrentRonak ShahNo ratings yet
- Chemical Effects of Electric Current - Class 8 - Notes - PANTOMATHDocument9 pagesChemical Effects of Electric Current - Class 8 - Notes - PANTOMATHsourav9823No ratings yet
- Electroplating for Amateurs: Classic Reference for Small WorkshopsFrom EverandElectroplating for Amateurs: Classic Reference for Small WorkshopsNo ratings yet
- Design and Stress Analysis of Marine Diesel Engine Piston Using Solid Works and Finite Element Method FEMDocument11 pagesDesign and Stress Analysis of Marine Diesel Engine Piston Using Solid Works and Finite Element Method FEMMahadi Hanna MridulNo ratings yet
- Structural Design of Buried Corrugated Polyethylene Pipes: R. K. M. EDocument9 pagesStructural Design of Buried Corrugated Polyethylene Pipes: R. K. M. EElvi PapajNo ratings yet
- Inspection Report Liquid Penetrant Examination For General StructuralDocument1 pageInspection Report Liquid Penetrant Examination For General StructuralJindarat KasemsooksakulNo ratings yet
- Rajasthan Technical University, Kota: SyllabusDocument1 pageRajasthan Technical University, Kota: SyllabusRitesh PatidarNo ratings yet
- Housing Quality Standards Guidebook 2013Document34 pagesHousing Quality Standards Guidebook 2013James PilcherNo ratings yet
- Zuellig MakatiDocument9 pagesZuellig MakatiMark DanielNo ratings yet
- Tall Vessels PDFDocument29 pagesTall Vessels PDFVenkatesh Sivarchana100% (1)
- Vpvo Vdo Catalogue 2019 06 enDocument66 pagesVpvo Vdo Catalogue 2019 06 enstarykNo ratings yet
- 00102W C G0 G000 PE SPC 0013 Rev 1 Standard Pipe SupportsDocument119 pages00102W C G0 G000 PE SPC 0013 Rev 1 Standard Pipe SupportsMurtadda MohammedNo ratings yet
- Mechanical Properties of MetalsDocument15 pagesMechanical Properties of MetalsLaurence Bande Del RosarioNo ratings yet
- WeatherBarriers PDFDocument15 pagesWeatherBarriers PDFcolawariNo ratings yet
- (MCQ'S) Properties of Matter & NDTDocument12 pages(MCQ'S) Properties of Matter & NDTarpit patilNo ratings yet
- 300 Industrial Structures PDFDocument70 pages300 Industrial Structures PDFcurvedbrainNo ratings yet
- RIT Pipe Labeling Painting and Valve Tag Standard 03 01 2013 PDFDocument3 pagesRIT Pipe Labeling Painting and Valve Tag Standard 03 01 2013 PDFThaiminh Vo100% (1)
- Cement ABCDocument100 pagesCement ABCSriram Ramanujam100% (1)
- Practices For Grounding and Bonding of Cable Trays - EEPDocument5 pagesPractices For Grounding and Bonding of Cable Trays - EEPSaraswatapalitNo ratings yet
- Optimizing Cooling Performance of A Data Center Using CFD Simulation and MeasurementsDocument8 pagesOptimizing Cooling Performance of A Data Center Using CFD Simulation and MeasurementsBrandon AtzNo ratings yet
- Operation Manual of AHUDocument17 pagesOperation Manual of AHUpiyushsingh7881020No ratings yet
- BS en 10088-4-2009Document48 pagesBS en 10088-4-2009khanhNo ratings yet
- Fatigue Ch12Document87 pagesFatigue Ch12Bolaji Suberu100% (1)
- Method To Determine Hot Permeability and Strength of Ceramic Shell MouldsDocument5 pagesMethod To Determine Hot Permeability and Strength of Ceramic Shell MouldsuzairmetallurgistNo ratings yet
- En 10297-1-2003Document46 pagesEn 10297-1-2003Mircea VladNo ratings yet
- Zamil Air Conditioners: Leading Manufacturer of HVAC Systems in the Middle EastDocument23 pagesZamil Air Conditioners: Leading Manufacturer of HVAC Systems in the Middle EastgagokapalaNo ratings yet
- PIPE LAYING PROJECT (Sent To Ms. Analyn) PDFDocument1 pagePIPE LAYING PROJECT (Sent To Ms. Analyn) PDFJamaica RolloNo ratings yet
- Selecting Cooling Coils Without Proprietary Software - Issue Oct-Dec 2002Document12 pagesSelecting Cooling Coils Without Proprietary Software - Issue Oct-Dec 2002dokundot100% (2)
- Punch Tonnage Chart: Tonnage Required To Punch Round Holes in ASTM A-36 Structural SteelDocument1 pagePunch Tonnage Chart: Tonnage Required To Punch Round Holes in ASTM A-36 Structural Steelsmartcad60No ratings yet
- Multiple Choice Question Material ScienceDocument2 pagesMultiple Choice Question Material Sciencemanish_agr8567% (3)
- Type PrecastDocument43 pagesType Precastruya mNo ratings yet
- 1 s2.0 S1537511005001510 MainDocument9 pages1 s2.0 S1537511005001510 MainGokul Goku SanthiNo ratings yet
- PSV LPG TankDocument8 pagesPSV LPG TankyosafatedenNo ratings yet