0% found this document useful (0 votes)
170 views23 pagesUnderstanding Iron Overload Disorders
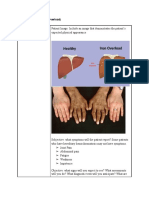
Iron overload occurs when there is excessive absorption of iron from the gastrointestinal tract or through regular blood transfusions, leading to an accumulation of iron in tissues that can damage organs. The most common causes of iron overload are hereditary hemochromatosis, transfusional iron overload from chronic anemias treated with blood transfusions, and African iron overload related to dietary iron intake. Iron chelation therapy uses drugs like deferasirox or deferiprone to promote the excretion of iron from the body and prevent organ damage.
Uploaded by
Kaylee NesbitCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
170 views23 pagesUnderstanding Iron Overload Disorders
Iron overload occurs when there is excessive absorption of iron from the gastrointestinal tract or through regular blood transfusions, leading to an accumulation of iron in tissues that can damage organs. The most common causes of iron overload are hereditary hemochromatosis, transfusional iron overload from chronic anemias treated with blood transfusions, and African iron overload related to dietary iron intake. Iron chelation therapy uses drugs like deferasirox or deferiprone to promote the excretion of iron from the body and prevent organ damage.
Uploaded by
Kaylee NesbitCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PPT, PDF, TXT or read online on Scribd