Professional Documents
Culture Documents
Kimia Inti: Nuclear Chemistry
Uploaded by
dimasaditya28Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kimia Inti: Nuclear Chemistry
Uploaded by
dimasaditya28Copyright:
Available Formats
1
KIMIA INTI
Nuclear Chemistry
2
Nuclear Equations
Nucleons: particles in the nucleus:
p
+
: proton
n
0
: neutron.
Mass number: the sume of the
number of p
+
and n
0
.
Atomic number: the number of p
+
.
Nuclear equations, the total number
of nucleons is conserved:
3
Sample Nuclear Equations
He Th U
4
2
234
90
238
92
+
4
2
He - o
particle
| +
0
1
14
7
14
6
N C
0
-1
| - |
particle
4
Three Types Of Decay
Processes
o-radiation
the loss of
4
2
He from the nucleus,
|-radiation
the loss of an electron from the nucleus,
-radiation
the loss of high-energy photon from the
nucleus.
5
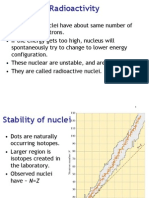
Radioactivity
6
Types of Radioactive Decay
Ensure
conservation of
nucleons
Write all particles
with their atomic
and mass numbers.
Nucleons can
undergo decay
e p n
1
0
1
1
0
1
+
n e p
0
1
1
0
1
1
+
| -particle emission
Electron capture
7
Types of Radioactive Decay
8
Neutron-to-Proton Ratio
The proton has high mass and high charge
proton-proton repulsion is large.
The cohesive forces in the nucleus are
called strong nuclear forces. Neutrons are
involved with the strong nuclear force.
As more protons are added (the nucleus
gets heavier) the proton-proton repulsion
gets larger.
9
Radioactive Series
A nucleus usually undergoes more
than one transition on its path to
stability.
The series of nuclear reactions that
accompany this path is the
radioactive series.
Nuclei resulting from radioactive
decay are called daughter nuclei.
10
An Example Radioactive
Decay Series
11
Nuclear Transmutations
Nuclear transmutations are the
collisions between nuclei.
14
N +
4
o
17
O +
1
H.
The above reaction is written in
short-hand notation:
14
N(o,p)
17
O.
To overcome electrostatic forces,
charged particles need to be
accelerated before they react.
12
Nuclear Transmutations
13
Radioactive Half-Lives
90
Sr has a half-life of 28.8 yr.
90
38
Sr
90
39
Y +
0
-1
e
Each isotope has a characteristic half-life.
Half-lives are not affected by temperature,
pressure or chemical composition.
Natural radioisotopes tend to have longer
half-lives than synthetic radioisotopes.
14
Rates of Radioactive Decay
15
Rates of Radioactive Decay
16
Carbon Dating
Carbon-14 is used to
determine the ages of
organic compounds
We assume the ratio of
12
C to
14
C has been
constant over time.
For us to detect
14
C
the object must be
less than 50,000 years
old.
The half-life of
14
C is
5,730 years.
| +
0
1
14
7
14
6
N C
17
kt
N
N
ln
0
t
=
(
Rates of Radioactive Decay
Radioactive decay is a first order process:
Rate = kN
N the number of
radionuclides
k the first order rate
constant
18
Detection of Radioactivity
19
6
14
CO
2
+ 6H
2
O
14
C
6
H
12
O
6
+ 6O
2
sunlight
chlorophyll
Radiotracers
Radiotracers are used to follow an
element through a chemical
reaction.
Photosynthesis has been studied
using
14
C:
The carbon dioxide is said to be
14
C
labeled.
20
Einstein showed that mass and energy are
proportional:
E = mc
2
The mass of a nucleus is less than the
mass of their nucleons. the mass defect!
Binding energy is the energy required to
separate a nucleus into its nucleons.
Since E = mc
2
the binding energy is
related to the mass defect.
Energy Changes in Nuclear
Reactions
21
Nuclear Binding Energies
22
Nuclear Fission
Splitting of heavy nuclei is
exothermic for large mass numbers.
Consider a neutron bombarding a
235
U nucleus:
23
A Nuclear Fission Process
24
Chain Reactions
The number of fissions and the
energy increase rapidly - eventually,
a chain reaction forms.
The minimum mass of fissionable
material is required for a chain
reaction critical mass.
25
The Fission Process
26
The Fission Process
For subcritical masses, the neutrons
escape and no chain reaction occurs.
At critical mass, the chain reaction
accelerates.
Anything over critical mass is called
supercritical mass.
Critical mass for
235
U is about 1 kg.
27
Atomic Bombs
28
Nuclear Reactors
Use a subcritical mass of
235
U (enrich
238
U with
about 3%
235
U)
Enriched
235
UO
2
pellets
are encased in Zr or
stainless steel rods.
Control rods are
composed of Cd or B,
which absorb neutrons.
Moderators are inserted
to slow down the
neutrons.
Natural abundance
uranium used as a
fuel souce.
Enriched
235
UO
2
pellets are encased in
Zr rods.
Heavy water is used
as the moderator and
the coolant.
Heat produced in the reactor core is removed by a
cooling fluid to a large tank of water (producing
steam). Steam drives an electric generator.
29
A Schematic Nuclear
Reactor
30
Nuclear Fusion
Light nuclei can fuse to form heavier nuclei.
Most reactions in the Sun are fusion.
Fusion products are not usually radioactive, so
fusion is a good energy source.
Also, the hydrogen required for reaction can
easily be supplied by seawater.
However, high energies are required to overcome
repulsion between nuclei before reaction can
occur.
High energies are achieved by high
temperatures: the reactions are thermonuclear.
31
Fusion of tritium and deuterium requires about
40,000,000K:
2
1
H +
3
1
H
4
2
He +
1
0
n
These temperatures can be achieved in a nuclear
bomb or a tokamak.
A tokamak is a magnetic bottle: strong magnetic
fields contained a high temperature plasma so
the plasma does not come into contact with the
walls. (No known material can survive the
temperatures for fusion.)
To date, about 3,000,000 K has been achieved in
a tokamak.
Nuclear Fusion
32
Biological Effects of
Radiation
The penetrating power of radiation is
a function of mass.
-radiation (zero mass) penetrates
deeply
|-radiation penetrates much further
than o-radiation
Radiation absorbed by tissue causes
excitation (nonionizing radiation) or
ionization (ionizing radiation).
Ionizing radiation is much more
harmful than nonionizing radiation.
33
Biological Effects of
Radiation
34
Biological Effects of
Radiation
Most ionizing radiation interacts with
water in tissues to form H
2
O
+
.
The H
2
O
+
ions react with water to
produce H
3
O
+
and OH.
OH has one unpaired electron. It is
called the hydroxy radical.
Free radicals generally undergo chain
reactions.
35
The SI unit for radiation is the becquerel
(Bq).
1 Bq is one disintegration per second.
The curie (Ci) is 3.7 10
10
disintegrations
per second. (Rate of decay of 1 g of Ra.)
Absorbed radiation is measured in the
gray (1 Gy is the absorption of 1 J of
energy per kg of tissue) or the radiation
absorbed dose (1 rad is the absorption of
10
-2
J of radiation per kg of tissue).
Radiation Doses
36
The Relative Biological
Effectiveness
Not all forms of radiation have the same
effect,
Account for the differences using RBE
(relative biological effectiveness
~ for |- and -radiation and 10 for o radiation).
rem (roentgen equivalent for man) =
rads.RBE
SI unit for effective dosage is the Sievert
(1Sv = RBE.1Gy = 100 rem).
37
Radiation Doses
38
Radon
The nucleus
222
86
Rn is a product of
238
92
U.
Radon exposure accounts for more than
half the 360 mrem annual exposure to
ionizing radiation.
Rn is a noble gas so is extremely stable.
The half-life of is 3.82 days.
It decays as follows:
222
86
Rn
218
84
Po +
4
2
He
39
Biological Effects of Radon
The o-particles produced have a high RBE.
Therefore, inhaled Rn is thought to cause lung
cancer.
The picture is complicated by realizing that
218
Po
has a short half-life (3.11 min) also:
218
84
Po
214
82
Pb +
4
2
He
The
218
Po gets trapped in the lungs where it
continually produces o-particles.
The EPA recommends
222
Rn levels in homes to be
kept below 4 pCi per liter of air.
You might also like
- Practice Makes Perfect in Chemistry: Nuclear Chemistry with AnswersFrom EverandPractice Makes Perfect in Chemistry: Nuclear Chemistry with AnswersRating: 5 out of 5 stars5/5 (1)
- Chapter 23: Nuclear Chemistry: - Nuclear Changes - Nuclear Equations - U TH+ HeDocument72 pagesChapter 23: Nuclear Chemistry: - Nuclear Changes - Nuclear Equations - U TH+ HeNancy SantosNo ratings yet
- Nuclear LectureDocument35 pagesNuclear LectureRonalson SiraitNo ratings yet
- Practice Makes Perfect in Chemistry: Nuclear EnergyFrom EverandPractice Makes Perfect in Chemistry: Nuclear EnergyRating: 5 out of 5 stars5/5 (1)
- Lecture 3 Nuclear ChemistryDocument52 pagesLecture 3 Nuclear ChemistryGuilbert FajardoNo ratings yet
- Student CH 19 NuclearDocument34 pagesStudent CH 19 NuclearMaheshNo ratings yet
- Nuclear ChemistryDocument42 pagesNuclear ChemistryJann Romene Decena100% (1)
- Nuclear PhysicsDocument24 pagesNuclear PhysicsCalvin LabialNo ratings yet
- Chem 114-Nuclear ChemistryDocument36 pagesChem 114-Nuclear ChemistryKaizNo ratings yet
- Nuclear ChemistryDocument33 pagesNuclear ChemistryPRIYA ManobanNo ratings yet
- 2-Basic Concepts of Nuclear Physics and Overview of ReactorDocument67 pages2-Basic Concepts of Nuclear Physics and Overview of ReactorswamikashyapNo ratings yet
- Nuclear Chemistry and Applications of RadioactivityDocument15 pagesNuclear Chemistry and Applications of RadioactivityAnusha KhadkaNo ratings yet
- 6 CH 19 Nuclear ChemistryDocument35 pages6 CH 19 Nuclear ChemistryFatin IziantiNo ratings yet
- Nuclear ChemistryDocument78 pagesNuclear Chemistrynagendra_rdNo ratings yet
- UNIT 5 NcesDocument100 pagesUNIT 5 NcesSHAIKJAFARNo ratings yet
- Nuclear ChemistryDocument18 pagesNuclear ChemistryloeyyeolNo ratings yet
- Miss Rabia Najam: Nuclear PHYSICS Presentation Submitted ToDocument13 pagesMiss Rabia Najam: Nuclear PHYSICS Presentation Submitted ToWajeeha IftikharNo ratings yet
- Radioactivity NotesDocument16 pagesRadioactivity NotesNooruddin SheikNo ratings yet
- CHEM 357 - 24 - Lecture 5-6Document33 pagesCHEM 357 - 24 - Lecture 5-6Abede Saviour DelaliNo ratings yet
- Nuclear Chemistry and Applications of RadioactivityDocument7 pagesNuclear Chemistry and Applications of RadioactivityshikshitdheroNo ratings yet
- Lecture 5Document18 pagesLecture 5Marielle BrionesNo ratings yet
- Nuclear ChemistryDocument47 pagesNuclear ChemistryEmman Revilla100% (3)
- Chapter 5 NuclearDocument21 pagesChapter 5 NuclearUrooj GulNo ratings yet
- UntitledDocument36 pagesUntitledahsan shahNo ratings yet
- Notes On Nuclear ChemistryDocument1 pageNotes On Nuclear Chemistryanon_2475374No ratings yet
- Chapter 22Document43 pagesChapter 22Mohamed Saied FrahatNo ratings yet
- Nuclear Chemistry: Lecture PresentationDocument39 pagesNuclear Chemistry: Lecture Presentationvibhakar4uNo ratings yet
- CH 15Document53 pagesCH 15Ronalson SiraitNo ratings yet
- 4 Nuclear Chemistry (Type of Radioactive Decay)Document15 pages4 Nuclear Chemistry (Type of Radioactive Decay)Fatin IziantiNo ratings yet
- Physics 10 ExamDocument27 pagesPhysics 10 ExamPreston LimeNo ratings yet
- Atomic Nucleus and RadioactivityDocument59 pagesAtomic Nucleus and Radioactivitymladen lakic100% (2)
- Chapter 22 Nuclear Chem Study GuideDocument5 pagesChapter 22 Nuclear Chem Study GuideVicky100% (2)
- Nuclear EnergyDocument12 pagesNuclear EnergyOjashwi SharmaNo ratings yet
- Chapter 2 Nuclear ChemistryDocument26 pagesChapter 2 Nuclear ChemistryJakeNK94No ratings yet
- Nuclear EnergyDocument25 pagesNuclear EnergyMohab GarawanyNo ratings yet
- Nuclear and Radiation 100LDocument12 pagesNuclear and Radiation 100Lekanadefestus007No ratings yet
- RadioactivityDocument31 pagesRadioactivityRamliRemNo ratings yet
- Radioactivity and X-Ray ProductionDocument46 pagesRadioactivity and X-Ray ProductionEkwama EwugaNo ratings yet
- RadioactivityDocument31 pagesRadioactivitysanaNo ratings yet
- Chapter 07-Nuclear EnergyDocument57 pagesChapter 07-Nuclear EnergyfadlunisaNo ratings yet
- First Year Chemistry CHEM1901/1903: The Nucleus Lectures 1-5 Molecular Structure Lectures 16-18Document13 pagesFirst Year Chemistry CHEM1901/1903: The Nucleus Lectures 1-5 Molecular Structure Lectures 16-18BornKimNo ratings yet
- LecPPT3 - Neutron Interactions, Cross-Section and ExamplesDocument32 pagesLecPPT3 - Neutron Interactions, Cross-Section and ExamplesVishal MeenaNo ratings yet
- Nuclear Power Plants PDFDocument112 pagesNuclear Power Plants PDFVadiraj PatilNo ratings yet
- Nuclear Decay Applications of Nuclear PhysicsDocument19 pagesNuclear Decay Applications of Nuclear PhysicspfmlerNo ratings yet
- 13 ChangesInTheNucleus 2bDocument14 pages13 ChangesInTheNucleus 2bmainethemaineNo ratings yet
- 03 - Radioactivity and Nuclear Transformation - Page No. 167Document6 pages03 - Radioactivity and Nuclear Transformation - Page No. 167Arvind TiwariNo ratings yet
- Revision Notes For NucleationDocument7 pagesRevision Notes For Nucleationsumakodipaka938No ratings yet
- Group3 Rad106Document50 pagesGroup3 Rad106chacha 7074684No ratings yet
- Nuclear Reactions by UlfatDocument20 pagesNuclear Reactions by Ulfataiman javaidNo ratings yet
- Nuclear Reactions: William L Masterton Cecile N. Hurley Edward J. NethDocument68 pagesNuclear Reactions: William L Masterton Cecile N. Hurley Edward J. NethJulieAnne DelaCruzNo ratings yet
- Radioactivity NotesDocument3 pagesRadioactivity Notessreevaishnava01No ratings yet
- Nuclear Engineering 2Document35 pagesNuclear Engineering 2rajeshNo ratings yet
- Module 1 - Basics of Biological Effects of Ionizing RadiationDocument30 pagesModule 1 - Basics of Biological Effects of Ionizing RadiationAndrés PacompíaNo ratings yet
- Chapter 5 RadiometryDocument25 pagesChapter 5 RadiometrytufabededaNo ratings yet
- Radioisotope TechniquesDocument63 pagesRadioisotope TechniquesPallavi Kanungo UpritNo ratings yet
- Fridays LessonDocument13 pagesFridays Lessonapi-239941033No ratings yet
- Nuclear DecayDocument34 pagesNuclear DecayAyya ChannNo ratings yet
- Quantum Mechanics PresentationDocument31 pagesQuantum Mechanics Presentationemad11518100% (1)
- 2 - Nuc ChemDocument44 pages2 - Nuc ChemRENIER JANE GAIDNo ratings yet
- Gamma Rays: Syahir Mansor, PHD Ippt, UsmDocument52 pagesGamma Rays: Syahir Mansor, PHD Ippt, UsmLailatul KisstinaNo ratings yet
- Criteria For Radionuclide Activity Concentrations For Food and Drinking Water IAEADocument63 pagesCriteria For Radionuclide Activity Concentrations For Food and Drinking Water IAEAabdulaziz saadNo ratings yet
- Fukushima Radioactive Water MDocument2 pagesFukushima Radioactive Water MYana PopovaNo ratings yet
- (OTHER) QC in Production of Radiopharmaceuticals PDFDocument150 pages(OTHER) QC in Production of Radiopharmaceuticals PDFMark DenhamNo ratings yet
- ARC-100 Technical Overview August 23 - FINALDocument42 pagesARC-100 Technical Overview August 23 - FINALIanNo ratings yet
- JU PresentationDocument12 pagesJU PresentationarefinnshiblyyNo ratings yet
- Radioactive PollutionDocument4 pagesRadioactive PollutionAgnivesh SharmaNo ratings yet
- Effects of Radiation To AgricultureDocument3 pagesEffects of Radiation To AgricultureShairaManauisNo ratings yet
- Nuclear FusionDocument11 pagesNuclear Fusiondo4 humanNo ratings yet
- 01 - NPP Instrumentation Systems Handbook 1Document318 pages01 - NPP Instrumentation Systems Handbook 1Ryando HutagalungNo ratings yet
- ISOASTM51204Document10 pagesISOASTM51204Partth VachhaniNo ratings yet
- Chapter 23 Nuclear ChemistryDocument44 pagesChapter 23 Nuclear ChemistryArienta NiadisyaNo ratings yet
- Achytherapy 3HAXAPDocument464 pagesAchytherapy 3HAXAPIkang FauziNo ratings yet
- Atomic Structure Knowledge Organiser - Foundation and HigherDocument2 pagesAtomic Structure Knowledge Organiser - Foundation and HigheranqelineeNo ratings yet
- AzSPU Procedure For Transportation of Radioactive MaterialsDocument24 pagesAzSPU Procedure For Transportation of Radioactive MaterialsAmir M. ShaikhNo ratings yet
- Lesson 6. Henry-Moseley-the-Atomic-Number HO PDFDocument14 pagesLesson 6. Henry-Moseley-the-Atomic-Number HO PDFValerie Amor SalabaoNo ratings yet
- Physics Notes Radioactivity & Astronomy-2Document12 pagesPhysics Notes Radioactivity & Astronomy-2Arshad HossainNo ratings yet
- Appendix E, 'External Dose Estimates From NTS Fallout'Document57 pagesAppendix E, 'External Dose Estimates From NTS Fallout'scribd2100% (5)
- Brest Od 300Document2 pagesBrest Od 300Abdalrahman Taha (Jíɾaïya)No ratings yet
- 1 RT08 Occupational Exposure WEBDocument87 pages1 RT08 Occupational Exposure WEBRichar Pinedo CadilloNo ratings yet
- Criticality Detection at UK Nuclear Licenced SitesDocument23 pagesCriticality Detection at UK Nuclear Licenced SitesEm GerNo ratings yet
- An Investigation of Electrostatically Deposited Radionuclides On A Latex Balloon - Final-WITH REVISIONSDocument5 pagesAn Investigation of Electrostatically Deposited Radionuclides On A Latex Balloon - Final-WITH REVISIONSYerrit PriceNo ratings yet
- Preservation of Cowpea Against Callosobruchus Maculatus Using Gamma-IrradiationDocument3 pagesPreservation of Cowpea Against Callosobruchus Maculatus Using Gamma-IrradiationInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Chem 86 Nuclear ChemistryDocument1 pageChem 86 Nuclear ChemistryHernan SalvadorNo ratings yet
- Nuclear Chemistry Problems-1Document4 pagesNuclear Chemistry Problems-1Marques CatheyNo ratings yet
- Pakistan Nuclear Regulatory AuthorityDocument4 pagesPakistan Nuclear Regulatory AuthorityAdina RehmanNo ratings yet
- Script & Summary of Marie CurieDocument2 pagesScript & Summary of Marie CurieGrace Victoria Poo 1428041No ratings yet
- Video Guide White Light Black Rain Destruction of Hiroshima Nagasaki - Full Video GuideDocument2 pagesVideo Guide White Light Black Rain Destruction of Hiroshima Nagasaki - Full Video GuideAws FadhilNo ratings yet
- SARA UserManual 2 6rev0Document122 pagesSARA UserManual 2 6rev0Roberto Hijosa GarciaNo ratings yet
- Radiochemistry and RadiometryDocument20 pagesRadiochemistry and RadiometryAmanuel Maru100% (2)