Professional Documents
Culture Documents
VSEPR Theory Bond Angle Table
Uploaded by
Audrey HizonOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
VSEPR Theory Bond Angle Table
Uploaded by
Audrey HizonCopyright:
Available Formats
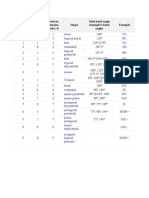
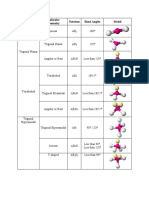
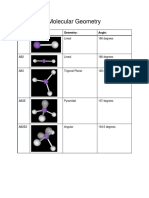
VSEPR Table
The bond angles in the table below are ideal angles from the simple VSEPR theory, followed by the actual
angle for the example given in the following column where this differs. For many cases, such as trigonal
pyramidal and bent, the actual angle for the example differs from the ideal angle, but all examples differ by
different amounts. For example, the angle in H2S (92°) differs from the tetrahedral angle by much more than
the angle for H2O (104.5°) does.
Bonding Ideal Bond Angle
Lone Electron
Electron Pairs Shape (example's bond Example Image
Pairs Domains
angle)
2 0 2 linear 180° CO2
3 0 3 trigonal planar 120° BF3
2 1 3 bent 120° (119°) SO2
4 0 4 tetrahedral 109.5° CH4
trigonal
3 1 4 109.5° (107.5°) NH3
pyramidal
2 2 4 angular 109.5° (104.5°) H2 O
trigonal
5 0 5 90°, 120° PCl5
bipyramidal
180°, 120°, 90° (173.1°,
4 1 5 seesaw SF4
101.6°)
3 2 5 T-shaped 90°, 180° (87.5°, < 180°) ClF3
2 3 5 linear 180° XeF2
6 0 6 octahedral 90°, 180° SF6
square
5 1 6 90° (84.8°), 180° BrF5
pyramidal
4 2 6 square planar 90° 180° XeF4
pentagonal
7 0 7 90°, 72° IF7
bipyramidal
You might also like
- Chemical Bonds ExplainedDocument10 pagesChemical Bonds ExplainedNor Fairul Bin SudarmanNo ratings yet
- Science 9 Electricity QuizDocument6 pagesScience 9 Electricity Quizapi-265758110100% (2)
- HL Topic 7 17 EquilibriumDocument9 pagesHL Topic 7 17 EquilibriumDavid DancerNo ratings yet
- Atomic Structure NotesDocument9 pagesAtomic Structure Notescgao30No ratings yet
- AP Chemistry 2008 MC QuestionsDocument21 pagesAP Chemistry 2008 MC QuestionsJoanna IpNo ratings yet
- Revise Chemistry PDFDocument11 pagesRevise Chemistry PDFAli AshrafNo ratings yet
- Algebraic Method To Balance Chemical EquationDocument3 pagesAlgebraic Method To Balance Chemical EquationBruce WalkerNo ratings yet
- HighSchool-Chemistry G10 To G12Document444 pagesHighSchool-Chemistry G10 To G12patkhsheng@hotmail.com100% (1)
- Tutorial 5 Equilibrium AnswerDocument4 pagesTutorial 5 Equilibrium AnswerNor AishahNo ratings yet
- Chemistry Periodic Trends ActivityDocument6 pagesChemistry Periodic Trends ActivityocNo ratings yet
- IA - Activation EnergyDocument6 pagesIA - Activation Energy14nganhc1No ratings yet
- Cap 1 - Electrochemistry (Hardcover) by Carl H. Hamann, Andrew HamnettDocument12 pagesCap 1 - Electrochemistry (Hardcover) by Carl H. Hamann, Andrew Hamnettflavyma25No ratings yet
- Born-Haber CycleDocument5 pagesBorn-Haber CycleShahnaz AhmedNo ratings yet
- SAT II Chemistry Study Guide Pt. 1Document10 pagesSAT II Chemistry Study Guide Pt. 1Caryn Tran100% (4)
- Radioactive Dating - Half-Life WS PDFDocument2 pagesRadioactive Dating - Half-Life WS PDFb RkNo ratings yet
- Biology 9700 Notes Topic 16 Inherited Change 2019-20Document32 pagesBiology 9700 Notes Topic 16 Inherited Change 2019-20ADEEL AHMAD67% (3)
- In-Class Worksheet AnswersDocument6 pagesIn-Class Worksheet AnswersalgonzNo ratings yet
- Bond Enthalpy WorksheetDocument6 pagesBond Enthalpy WorksheetTanisha DamleNo ratings yet
- Basic Electricity Questions IGCSEDocument11 pagesBasic Electricity Questions IGCSEFan Xin Foo100% (1)
- Chemistry Ia TemplateDocument5 pagesChemistry Ia Templateapi-546066323No ratings yet
- Naming Ionic Compounds WorksheetDocument3 pagesNaming Ionic Compounds Worksheetgowrimanohar1975No ratings yet
- 2-Chem 1101 The The Properties of Gases & Solutions (Text)Document55 pages2-Chem 1101 The The Properties of Gases & Solutions (Text)Tmmp SmileNo ratings yet
- Singhania University BSC Aircraft Maintenance Engineering Vi Sem Model Question PaperDocument3 pagesSinghania University BSC Aircraft Maintenance Engineering Vi Sem Model Question PaperKartick RoyNo ratings yet
- 8.4 (147 Marks) : MarkschemeDocument65 pages8.4 (147 Marks) : MarkschemeSemwezi EnockNo ratings yet
- IGCSE Chemistry - Types of Chemical BondsDocument7 pagesIGCSE Chemistry - Types of Chemical BondsdanielmahsaNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- Introduction To MolecularDocument1 pageIntroduction To Molecularclairole quilantangNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Molecular Geometry Unit 02Document26 pagesMolecular Geometry Unit 02Iqra BaigNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryDean Joyce Alboroto100% (1)
- MoleDocument2 pagesMoleapi-233333580No ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Electron Bonding Molecular Geometry GuideDocument2 pagesElectron Bonding Molecular Geometry GuideRichamille Ann RicaforteNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- List of Molecular GeometryDocument1 pageList of Molecular GeometryEsmeNo ratings yet
- List of Molecular GeometryDocument1 pageList of Molecular GeometryEsmeNo ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Properties of 2D Shapes - AnswersDocument1 pageProperties of 2D Shapes - Answerscloud scapeNo ratings yet
- Regular Polygons TableDocument2 pagesRegular Polygons TableGrace HutallaNo ratings yet
- Finding Angles by Degrees: Answer KeyDocument2 pagesFinding Angles by Degrees: Answer KeySharmila ShajahanNo ratings yet
- Vsepr ChartDocument2 pagesVsepr Chartapi-239855791No ratings yet
- Class: Xi Inorganic Chemistry DPP. NO.-9Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-9Radhika MohataNo ratings yet
- Chapter 3 Polygons2Document26 pagesChapter 3 Polygons2Jonard G. TrajanoNo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles BubblesNo ratings yet
- UT2 Worksheet - 2 KeyanswersDocument5 pagesUT2 Worksheet - 2 KeyanswersPushpalataNo ratings yet
- Class: Xi Inorganic Chemistry DPP. NO.-8Document2 pagesClass: Xi Inorganic Chemistry DPP. NO.-8Radhika MohataNo ratings yet
- Molecular GeometryDocument4 pagesMolecular Geometryapi-449127308No ratings yet
- Digital Text BookDocument12 pagesDigital Text BookBindu Vinu100% (1)
- BETE - TF-metric SPIRAL FULL CONEDocument2 pagesBETE - TF-metric SPIRAL FULL CONEOnie Hammamz Oyl100% (1)
- Ficha Tecnica Boquillas AspersionDocument1 pageFicha Tecnica Boquillas AspersionCesar Muñoz OssesNo ratings yet
- Geometry Quick Guide 1: Angles: Angle Types Angle RulesDocument1 pageGeometry Quick Guide 1: Angles: Angle Types Angle RulesROSLINA BINTI ABDUL RASHID MoeNo ratings yet
- Circle theorems review answers and exercisesDocument1 pageCircle theorems review answers and exercisesSiyah HashTagNo ratings yet
- 1 2 PDFDocument2 pages1 2 PDF祁伟No ratings yet
- Rasco - MV - Medium Velocity Directional Spray Nozzle - B.106-Aug21Document39 pagesRasco - MV - Medium Velocity Directional Spray Nozzle - B.106-Aug21Ary SetiawanNo ratings yet
- 6.1 - Radian Measure and Arc Length Math 30-1Document14 pages6.1 - Radian Measure and Arc Length Math 30-1Math 30-1 EDGE Study Guide Workbook - by RTD LearningNo ratings yet
- Lone Pairs of Central AtomDocument2 pagesLone Pairs of Central AtomChup KarNo ratings yet
- Geometry Teacher - S Skills Practice Chapter 4 PDFDocument34 pagesGeometry Teacher - S Skills Practice Chapter 4 PDFJamie BaczewskiNo ratings yet
- 7 Resonance Structure Ans PDFDocument3 pages7 Resonance Structure Ans PDFKuo SarongNo ratings yet
- Atomic Structure HL Multiple Choice Questions AnswersDocument3 pagesAtomic Structure HL Multiple Choice Questions AnswersMalak AlqaidoomNo ratings yet
- FT-IR and NMR Spectroscopic Studies of Salicylic Acid Derivatives. II. Comparison of 2-Hydroxy - and 2,4 - and 2,5-Dihydroxy DerivativesDocument15 pagesFT-IR and NMR Spectroscopic Studies of Salicylic Acid Derivatives. II. Comparison of 2-Hydroxy - and 2,4 - and 2,5-Dihydroxy DerivativesAdrielNo ratings yet
- Solving Problems by NMR SpectroscopyDocument16 pagesSolving Problems by NMR SpectroscopySiddarth PalletiNo ratings yet
- Atomic SizeDocument2 pagesAtomic SizeFozia's Beauty Tips & Entertainment FoziaNo ratings yet
- Effective Ionic Radii in Oxides and Fluorides : ReferencesDocument22 pagesEffective Ionic Radii in Oxides and Fluorides : ReferencesMoad BarbariNo ratings yet
- AnskjhbljsDocument7 pagesAnskjhbljsmillinagi95No ratings yet
- EsrDocument13 pagesEsrChiranjeevi TulluriNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- Bonding in Polyatomic Molecules: TopicsDocument44 pagesBonding in Polyatomic Molecules: TopicsAcikaNo ratings yet
- Teoría Del Funcional Densidad para Metales de Transición (Proser Chris Cramer)Document60 pagesTeoría Del Funcional Densidad para Metales de Transición (Proser Chris Cramer)ANDERSON RUBIO CLEVESNo ratings yet
- Teori Medan Ligan Menjelaskan Pembentukan Senyawa KompleksDocument42 pagesTeori Medan Ligan Menjelaskan Pembentukan Senyawa KompleksAnisa NurjanahNo ratings yet
- Symmetry and Group TheoryDocument24 pagesSymmetry and Group Theorywicki0007100% (1)
- Mössbauer Spectroscopy: Advanced Inorganic Chemistry SeminarDocument21 pagesMössbauer Spectroscopy: Advanced Inorganic Chemistry SeminarJose GalvanNo ratings yet
- Effects of Ligand Concentration on Coordination Compound PropertiesDocument28 pagesEffects of Ligand Concentration on Coordination Compound PropertiesCriztIan GgomesNo ratings yet
- Food Colorant Lab ReportDocument12 pagesFood Colorant Lab ReportJuanNo ratings yet
- Periodic Properties and Trends PDFDocument20 pagesPeriodic Properties and Trends PDFShivansh Awasthi XII B2No ratings yet
- 2016 2017 6 7 Notes QuantumDocument85 pages2016 2017 6 7 Notes QuantumAlexander AdrogueNo ratings yet
- 1H NMR SpectrosDocument84 pages1H NMR Spectrosapi-3723327100% (5)
- Classifying Solids and Understanding Semiconductor Band Theory (40 CharactersDocument58 pagesClassifying Solids and Understanding Semiconductor Band Theory (40 CharactersTanvi SuryawanshiNo ratings yet
- R K Sharma Coordination Chemistry 10-30Document23 pagesR K Sharma Coordination Chemistry 10-30Kamal KishoreNo ratings yet
- DAV PUBLIC SCHOOL'S GUIDE TO COORDINATION COMPOUNDSDocument36 pagesDAV PUBLIC SCHOOL'S GUIDE TO COORDINATION COMPOUNDSAnindya BhattacharyaNo ratings yet
- ლექცია.5- კომპლექსური-ნაერთებიDocument6 pagesლექცია.5- კომპლექსური-ნაერთებიNini TkavadzeNo ratings yet
- Atomic Emission SpectrosDocument18 pagesAtomic Emission Spectrosmatin5No ratings yet
- XPS & UPS Presentation at Addis Ababa UniversityDocument37 pagesXPS & UPS Presentation at Addis Ababa UniversityGuru P MNo ratings yet
- Identifying Elements Through Spectral Analysis: Andrew SpinelliDocument5 pagesIdentifying Elements Through Spectral Analysis: Andrew SpinelliAndrew SpinelliNo ratings yet
- Review On PhotoconductivityDocument6 pagesReview On PhotoconductivitySumon DebnathNo ratings yet
- E9 AtqDocument3 pagesE9 AtqAljan TabsNo ratings yet