Professional Documents
Culture Documents
Schaeffler Diagram
Uploaded by
Tridib DeyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Schaeffler Diagram
Uploaded by
Tridib DeyCopyright:
Available Formats
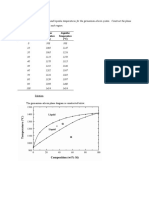
Application of the Schaeffler diagram
Schaeffler-diagram
30 28 0% Ferrite 26 Equivalent Nickel = %Ni + 30 x %C + 0,5 x %Mn 24 22 20 18 16 A+ M 14 12 10 Martensite 8 6 4 2 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 Equivalent Chrome = %Cr + %Mo + 1,5%Si + 0,5%Nb + 2% Ti F + M M+F A+M+F 100% 80% 40% 20% Austenite 10% 5%
Ferrite
A Schaeffler diagram can be used to represent the effect of the proportion of two elements (and therefore the composition of the alloy) on the structure obtained after rapid cooling from 1050C to room temperature. The figure shows that chromium is a ferrite stabilizer and nickel is an austenite stabiliser. This diagram shows the limits of the austenitic, ferritic and martensitic phases in relation to the chromium and nickel equivalent, calculated by using these expressions: Cr equivalent = (Cr)+2(Si)+1.5(Mo)+5(V)+5.5(Al)+1.75(Nb)+1.5(Ti)+0.75(W) Ni equivalent = (Ni)+(Co)+0.5(Mn)+0.3(Cu)+25(N)+30(C) with all concentrations being expressed in weight percentages. The Schaeffler diagram is an important tool for predicting the constitution of austenitic Cr-Ni steel welds with carbon contents up to 0.12%. However, it does not allow determination of the composition and volume of the carbide phase. Furthermore, for a carbon content lower than 0.12%, the agreement of predictions with actual system is reduced due to consumption of carbon by the carbide formation process. This Schaeffler diagram is especially suited to weld metals in order to predict the structure.
You might also like
- Schaeffler DiagramDocument9 pagesSchaeffler DiagramAji Ashiq75% (4)
- Ceramic Calculations Sample 3Document24 pagesCeramic Calculations Sample 3AkonSayagyiNo ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument4 pagesIron-Iron Carbide Phase Diagram ExampleWanda Andreas WidyatmokoNo ratings yet
- Vibrational Spectra of Organometallics: Theoretical and Experimental DataFrom EverandVibrational Spectra of Organometallics: Theoretical and Experimental DataNo ratings yet
- HW-6 Chap 9 Dan 10: ProblemsDocument6 pagesHW-6 Chap 9 Dan 10: ProblemsRifda Muthia AlvianaNo ratings yet
- Infrared Spectroscopy of Triatomics for Space ObservationFrom EverandInfrared Spectroscopy of Triatomics for Space ObservationNo ratings yet
- (演習課) 考古題重新打字 - Ch1 ~ Ch5Document5 pages(演習課) 考古題重新打字 - Ch1 ~ Ch5frank941214No ratings yet
- X-ray Absorption Spectroscopy for the Chemical and Materials SciencesFrom EverandX-ray Absorption Spectroscopy for the Chemical and Materials SciencesNo ratings yet
- 154614Document108 pages154614Danem HalasNo ratings yet
- Dasar Hitung Komposisi Taping %C (x100) : 5 %si (x100) : 2 Dan %MN (x100) : 10 Dan TRM: 0.040Document13 pagesDasar Hitung Komposisi Taping %C (x100) : 5 %si (x100) : 2 Dan %MN (x100) : 10 Dan TRM: 0.040Ratih Eka Mardhika SariNo ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet
- 104 PhaseDiags QS2AnsDocument6 pages104 PhaseDiags QS2Ansnilanga123No ratings yet
- Excel Implementation of Schaeffler and Delong Diagrams PDFDocument8 pagesExcel Implementation of Schaeffler and Delong Diagrams PDFEmad A.AhmadNo ratings yet
- Ainaya Febi Amalia - M0318008 - P2Document25 pagesAinaya Febi Amalia - M0318008 - P2Alifiananda Rahmatul Dafa KesumaNo ratings yet
- 1 PDFDocument1 page1 PDFYudi Candra BNo ratings yet
- 10.6 Continuous Cooling Transformation DiagramsDocument1 page10.6 Continuous Cooling Transformation DiagramsUlwan FianiNo ratings yet
- Alain Vignes-Extractive Metallurgy 2 - Metallurgical Reaction Processes-Wiley-IsTE (2011)Document6 pagesAlain Vignes-Extractive Metallurgy 2 - Metallurgical Reaction Processes-Wiley-IsTE (2011)hotdenNo ratings yet
- Result Analysis: Naoh EtDocument7 pagesResult Analysis: Naoh EtSuria Seri SulyanaNo ratings yet
- Schaffler DiagramDocument6 pagesSchaffler DiagramNikesh KoliNo ratings yet
- Hazem Raw Mix CompositionDocument11 pagesHazem Raw Mix CompositionHazem DiabNo ratings yet
- Bimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneDocument10 pagesBimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneTalitha AdhyaksantiNo ratings yet
- Experiment 1Document6 pagesExperiment 1Huda AmirNo ratings yet
- The Schaeffler and Delong DiagramsDocument3 pagesThe Schaeffler and Delong DiagramsMustafa SertNo ratings yet
- B-AD-030 Metal Dispersion Measurement of Precious Metal Supported Catalysts On Carbon enDocument4 pagesB-AD-030 Metal Dispersion Measurement of Precious Metal Supported Catalysts On Carbon ennattaphong.boonsrisodNo ratings yet
- Chapter 36 - Sulfur Compounds - Sparkman2011Document4 pagesChapter 36 - Sulfur Compounds - Sparkman2011elenitabastosNo ratings yet
- Composition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Document7 pagesComposition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Muhammad Ibkar YusranNo ratings yet
- HW SolutionsDocument16 pagesHW SolutionsoerbilNo ratings yet
- Universitas Indonesia: Mata Kuliah (Kode) Dosen Pengampu Tugas Nama MahasiswaDocument10 pagesUniversitas Indonesia: Mata Kuliah (Kode) Dosen Pengampu Tugas Nama MahasiswaHari AntoNo ratings yet
- Mohr Circle Questions (By Sadhu Singh)Document15 pagesMohr Circle Questions (By Sadhu Singh)HARSHWARDHAN SINGH SENGARNo ratings yet
- Chapter 9: Phase Diagrams: Issues To Address..Document38 pagesChapter 9: Phase Diagrams: Issues To Address..yunlu0705No ratings yet
- C4 Lab ReportDocument11 pagesC4 Lab ReportchaitanyaNo ratings yet
- TYPD ExercisesDocument10 pagesTYPD ExercisesConstance Lynn'da GNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument3 pagesIron-Iron Carbide Phase Diagram ExampleBenjamin Enmanuel Mango DNo ratings yet
- Chapter 10Document4 pagesChapter 10Izzat NasrullahNo ratings yet
- Engineering Materials - Manufacturing Engineering and TechnologyDocument12 pagesEngineering Materials - Manufacturing Engineering and Technologyhans vNo ratings yet
- Week 11Document12 pagesWeek 11lduran_63No ratings yet
- Data SheetDocument14 pagesData SheetMelchiNo ratings yet
- Grafik Hubungan Ca Vs Time (S) : Time (S) Ca Mol/liter LN Ca0/Ca 1/ca Ca0 Ca EndDocument4 pagesGrafik Hubungan Ca Vs Time (S) : Time (S) Ca Mol/liter LN Ca0/Ca 1/ca Ca0 Ca EndAbimata Dwi WahyuNo ratings yet
- Beginning Time T Time To Half Value of V: TheoreticalDocument6 pagesBeginning Time T Time To Half Value of V: TheoreticalAli Amir ShreifNo ratings yet
- MassSpectroscopy - Rule of 13Document45 pagesMassSpectroscopy - Rule of 13Maxi Ma100% (1)
- Hardenability of High CR White Cast IronDocument4 pagesHardenability of High CR White Cast IronanruloNo ratings yet
- Lab 1: Graphing Review: 1 Graph 1Document5 pagesLab 1: Graphing Review: 1 Graph 1Benson WangNo ratings yet
- EN380 Homework #6 Solution: 1084 C 1455 C 70% N i L + α C = 61% C = 73% W = = = 25% W = = = 75%Document4 pagesEN380 Homework #6 Solution: 1084 C 1455 C 70% N i L + α C = 61% C = 73% W = = = 25% W = = = 75%roseNo ratings yet
- Issues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..Document34 pagesIssues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..arif_ashraf94No ratings yet
- Escribes The Manufacture of Chlorine by The of Sodium Chloride Solution Using ADocument2 pagesEscribes The Manufacture of Chlorine by The of Sodium Chloride Solution Using ALuk HKNo ratings yet
- Phase DiagramDocument33 pagesPhase DiagramAlan TehNo ratings yet
- Artículo Just, Calculating Hardenability Curves.Document2 pagesArtículo Just, Calculating Hardenability Curves.fvc731No ratings yet
- Charge Mix Preparation During Steel MakingDocument11 pagesCharge Mix Preparation During Steel MakingVikram Mahajan100% (1)
- Mte360 W08 MT1Document2 pagesMte360 W08 MT1dholedaysNo ratings yet
- sm1 46Document2 pagessm1 46awoods12835No ratings yet
- 06 Diagram Phase PDFDocument24 pages06 Diagram Phase PDFTeknik PemesinanNo ratings yet
- Chapter 01 Temperature and Heat (PP 1-18)Document18 pagesChapter 01 Temperature and Heat (PP 1-18)Muhammad Ashfaq AhmedNo ratings yet
- Experiment 4 Binary Phase Diagrams 2022Document5 pagesExperiment 4 Binary Phase Diagrams 2022Rey DLRNo ratings yet
- Estudo Quantitativo de Materiais Cimentícios Por Difração de Raios XDocument12 pagesEstudo Quantitativo de Materiais Cimentícios Por Difração de Raios XHellen HeloyzeNo ratings yet
- Lamarsh Solutions Ch-3 Part1Document5 pagesLamarsh Solutions Ch-3 Part1mazhar100% (1)
- WPQ FormatDocument1 pageWPQ FormatTridib Dey100% (1)
- Radiography TestingDocument4 pagesRadiography TestingTridib DeyNo ratings yet
- Surface Cleaning and PaintingDocument3 pagesSurface Cleaning and PaintingTridib Dey100% (1)
- En9 070M55Document1 pageEn9 070M55Tridib DeyNo ratings yet
- JP STructural Steel WorksDocument3 pagesJP STructural Steel WorksTridib DeyNo ratings yet
- Hydrotest ProcedureDocument3 pagesHydrotest ProcedureTridib Dey88% (16)
- Hydrotest ProcedureDocument3 pagesHydrotest ProcedureTridib Dey88% (16)
- Itp PWHTDocument1 pageItp PWHTTridib DeyNo ratings yet
- Hydrotest ProcedureDocument3 pagesHydrotest ProcedureTridib Dey88% (16)
- DPTDocument3 pagesDPTTridib DeyNo ratings yet
- Is StandardsDocument8 pagesIs StandardsSasi KumarNo ratings yet
- Record For RT Procedure Qualification Record For Pipe Welding PDFDocument1 pageRecord For RT Procedure Qualification Record For Pipe Welding PDFTridib DeyNo ratings yet
- Conversion TableDocument1 pageConversion TableTridib DeyNo ratings yet
- Incoming Material ProcedureDocument2 pagesIncoming Material ProcedureTridib Dey100% (6)
- Welding VariablesDocument3 pagesWelding VariablesTridib DeyNo ratings yet
- PQR Is7307Document1 pagePQR Is7307Tridib Dey100% (1)
- Hardness Test ProcedureDocument2 pagesHardness Test ProcedureTridib Dey100% (2)
- 5 Whys PDFDocument5 pages5 Whys PDFTridib DeyNo ratings yet
- Hardness Test ProcedureDocument2 pagesHardness Test ProcedureTridib Dey100% (2)
- Pipe PWHTDocument3 pagesPipe PWHTTridib Dey0% (1)
- Welding ProcessesDocument7 pagesWelding ProcessesTridib DeyNo ratings yet
- PMIDocument2 pagesPMITridib DeyNo ratings yet
- Pipe ScheduleDocument1 pagePipe ScheduleTridib DeyNo ratings yet
- Alloy Steels - AISI DesignationsDocument2 pagesAlloy Steels - AISI DesignationsTridib DeyNo ratings yet
- Wps PQR Spec IndexDocument1 pageWps PQR Spec IndexTridib DeyNo ratings yet
- Arizona, Utah & New Mexico: A Guide to the State & National ParksFrom EverandArizona, Utah & New Mexico: A Guide to the State & National ParksRating: 4 out of 5 stars4/5 (1)
- New York & New Jersey: A Guide to the State & National ParksFrom EverandNew York & New Jersey: A Guide to the State & National ParksNo ratings yet
- South Central Alaska a Guide to the Hiking & Canoeing Trails ExcerptFrom EverandSouth Central Alaska a Guide to the Hiking & Canoeing Trails ExcerptRating: 5 out of 5 stars5/5 (1)
- Japanese Gardens Revealed and Explained: Things To Know About The Worlds Most Beautiful GardensFrom EverandJapanese Gardens Revealed and Explained: Things To Know About The Worlds Most Beautiful GardensNo ratings yet
- Naples, Sorrento & the Amalfi Coast Adventure Guide: Capri, Ischia, Pompeii & PositanoFrom EverandNaples, Sorrento & the Amalfi Coast Adventure Guide: Capri, Ischia, Pompeii & PositanoRating: 5 out of 5 stars5/5 (1)
- The Bahamas a Taste of the Islands ExcerptFrom EverandThe Bahamas a Taste of the Islands ExcerptRating: 4 out of 5 stars4/5 (1)