Professional Documents
Culture Documents
Tutorial 3
Uploaded by
Norzaifee NizamudinOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Tutorial 3
Uploaded by
Norzaifee NizamudinCopyright:
Available Formats
Faculty of Chemical Engineering
CHE553 Chemical Engineering Thermodynamics
TUTORIAL 3 1. A mixture containing equimolar amounts of benzene(1), toluene(2), and ethylbenzene(3) is flashed to conditions t/T = 110oC/383.15K and P = 90 kPa. Determine the equilibrium mole fraction {xi} and {yi} of the liquid and vapor phases formed and the molar fraction V of the vapor formed. Assume that Raoults law applies. A binary system of species 1 and 2 consists of vapor and liquid phases in equilibrium at temperature T. The overall mole fraction of species 1 in the system is z1 = 0.65. At temperature T, ln 1 = 0.67x22 P1sat = 32.27 kPa ln 2 = 0.67x12 P2sat = 73.14 kPa
2.
Assuming the validity of modified Raoults law, a) Over what range of pressures can this system exist as two phases at given T and z1? b) For liquid phase mole fraction x1 = 0.75, what is the pressure P and what molar fraction V of the system is vapor? c) Show whether or not the system exhibits an azeotrope. 3. n-butane is separated from an equimolar methane/n-butane gas mixture by compression of the gas to pressure P at 40oC (313.15 K). If 40% of the feed on a mole basis is condensed, what are pressure P and the resulting liquid and vapor compositions.
You might also like
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsFrom EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsRating: 5 out of 5 stars5/5 (1)
- CH 7 AssignmentDocument3 pagesCH 7 AssignmentUday Prakash SahuNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Assignment 1Document1 pageAssignment 1FatthulHadiNo ratings yet
- CH 7 Assignment PDFDocument3 pagesCH 7 Assignment PDFAftab57.No ratings yet
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeFrom EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNo ratings yet
- Thermodynamics TutorialDocument2 pagesThermodynamics TutorialMuhamad Hazim Zaaba0% (1)
- Vapor Liquid EquilibriumDocument39 pagesVapor Liquid EquilibriumTouhid IslamNo ratings yet
- CL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFDocument42 pagesCL-201 Chapter 6 Multiphase Systems (Compatibility Mode) PDFSuman MandalNo ratings yet
- Tutorial VIII IxDocument1 pageTutorial VIII IxZaid MansuriNo ratings yet
- ChE 101 VLE Practice ProblemsDocument2 pagesChE 101 VLE Practice ProblemsLester Jason T. ChengNo ratings yet
- 1-Vle Part 1Document30 pages1-Vle Part 1Arfa Zulkifli01No ratings yet
- Baylon, Et. Al.-Solving Problems Regarding Vapor-Liquid EquilibriumDocument21 pagesBaylon, Et. Al.-Solving Problems Regarding Vapor-Liquid EquilibriumblessaNo ratings yet
- VLEDocument38 pagesVLEIjal JaminNo ratings yet
- Lecture-8,9,10 VLE DiagramsDocument64 pagesLecture-8,9,10 VLE DiagramsShiavm PatelNo ratings yet
- Capitulo 1 Termoquimica 2Document41 pagesCapitulo 1 Termoquimica 2adrianaNo ratings yet
- Assignment 1 PDFDocument1 pageAssignment 1 PDFRoydia SimanNo ratings yet
- Solving Problems Regarding Vapor-Liquid EquilibriumDocument19 pagesSolving Problems Regarding Vapor-Liquid EquilibriumblessaNo ratings yet
- Introduction To Vapor Liquid EquilibriumDocument35 pagesIntroduction To Vapor Liquid Equilibriumshiv007anshNo ratings yet
- VleDocument42 pagesVleFatien ZakariaNo ratings yet
- Fluid Phase EquilibriaDocument19 pagesFluid Phase EquilibriaYli S'cNo ratings yet
- Elizalde Solis2005Document6 pagesElizalde Solis2005jasontodd22031995No ratings yet
- Introduction To Chemical Engineering ThermodynamicsDocument5 pagesIntroduction To Chemical Engineering ThermodynamicsCynosure SkyNo ratings yet
- Tutorial 1Document1 pageTutorial 1Norzaifee NizamudinNo ratings yet
- Tutorial 2Document1 pageTutorial 2AhmadAimanNo ratings yet
- Thermodynamics Question SetDocument10 pagesThermodynamics Question SetMaharghya BiswasNo ratings yet
- Che 415 2018-19 Part 3 PDFDocument66 pagesChe 415 2018-19 Part 3 PDFOsan ThorpeNo ratings yet
- Exam GuideDocument8 pagesExam GuideclaNo ratings yet
- Extra ProblemDocument2 pagesExtra ProblemradwaelhadadNo ratings yet
- Week 8-10Document66 pagesWeek 8-10fatthul hadi50% (2)
- CHE3161 - Semester1 - 2010 - SolutionsDocument14 pagesCHE3161 - Semester1 - 2010 - SolutionsvenkieeNo ratings yet
- Vapor Liquid EquilibriumDocument39 pagesVapor Liquid EquilibriumyeenNo ratings yet
- Chemical, Biochemical, and Engineering ThermodynamicsDocument37 pagesChemical, Biochemical, and Engineering Thermodynamicsdeepak pandeyNo ratings yet
- Ok Smith 2018 Chapter 3Document65 pagesOk Smith 2018 Chapter 3syayaj dhiniNo ratings yet
- Tutorial 3Document1 pageTutorial 3AnisAsyiqinNo ratings yet
- Energy Balnce For Unsteady State SystemsDocument39 pagesEnergy Balnce For Unsteady State SystemsAbdulRehman VirkNo ratings yet
- Week 2 - Vle Part 1Document35 pagesWeek 2 - Vle Part 1Syed Hassan Syed Hashim100% (1)
- Introduction To Phase Equilibria & SeparationsDocument30 pagesIntroduction To Phase Equilibria & SeparationsShawn ChanNo ratings yet
- ثرمو محاضرة 3 مرحلة 3Document38 pagesثرمو محاضرة 3 مرحلة 3Al-Hassan NeimaNo ratings yet
- Phase EquilibriaDocument14 pagesPhase EquilibriaPaden TranNo ratings yet
- Tutorial 1Document2 pagesTutorial 1Braham ChawlaNo ratings yet
- ثرمو2Document19 pagesثرمو2Al-Hassan NeimaNo ratings yet
- SCHX1014 - Chemical Engineering Thermodynamics - Unit 3Document17 pagesSCHX1014 - Chemical Engineering Thermodynamics - Unit 3Shanmuga PriyaNo ratings yet
- Phase Equilibria Two-Component System: I. Liquid-Liquid System Ideal SolutionDocument16 pagesPhase Equilibria Two-Component System: I. Liquid-Liquid System Ideal SolutionChelsea MartinezNo ratings yet
- Week 2 - Vle Part 1Document35 pagesWeek 2 - Vle Part 1dhanieemaNo ratings yet
- A Mixture Containing Equimolar Amounts of BenzeneDocument1 pageA Mixture Containing Equimolar Amounts of BenzeneFranciskus Peri0% (1)
- Worked ExamplesDocument5 pagesWorked ExamplesmpumelaqqNo ratings yet
- 7 1. Vapor Liquid EquilibriumDocument9 pages7 1. Vapor Liquid Equilibriumwaseemkhan49No ratings yet
- Advanced Thermodynamics: Vapor/Liquid EquilibriumDocument28 pagesAdvanced Thermodynamics: Vapor/Liquid Equilibriumdo_overNo ratings yet
- EC1 Problemario de Equilibrio de Fases y Calculos FlashDocument2 pagesEC1 Problemario de Equilibrio de Fases y Calculos FlashRosario Zambrano CoronaNo ratings yet
- PC2 - Practice Exam WorkedDocument12 pagesPC2 - Practice Exam WorkednomsyNo ratings yet
- I. Ternary SystemsDocument33 pagesI. Ternary SystemsCatriona BlackNo ratings yet
- Distillation TheoryDocument40 pagesDistillation TheoryIrvin HernandezNo ratings yet
- Vapor Liquid EquilibriumDocument39 pagesVapor Liquid EquilibriumJakeWilliam100% (1)
- 4.1 GasesDocument23 pages4.1 GasesVasanth Kumar BatumalaiNo ratings yet
- CH Be 3110 ProblemsDocument75 pagesCH Be 3110 ProblemsAnkit DhalNo ratings yet
- Chapter 3 - Phases and SolutionDocument66 pagesChapter 3 - Phases and SolutionSYUHADAFAATAHNo ratings yet
- Silva Oliver2001Document12 pagesSilva Oliver2001Moltimer Folchart CrawNo ratings yet
- Fundamentals of Gas LawsDocument9 pagesFundamentals of Gas LawsMangojuice MbeleNo ratings yet
- 2018u009 PDFDocument14 pages2018u009 PDFNorzaifee NizamudinNo ratings yet
- No Id Mac: Borang Penilaian Ujian BleepDocument1 pageNo Id Mac: Borang Penilaian Ujian BleepNorzaifee NizamudinNo ratings yet
- Surds Past Edexcel Exam Questions: C Studywell Publications Ltd. 2015Document4 pagesSurds Past Edexcel Exam Questions: C Studywell Publications Ltd. 2015Norzaifee NizamudinNo ratings yet
- 0580 m17 QP 42 PDFDocument20 pages0580 m17 QP 42 PDFNorzaifee NizamudinNo ratings yet
- Tips Service ChargesDocument7 pagesTips Service ChargesNorzaifee NizamudinNo ratings yet
- Bukit Tinggi Booking Number: 990110 Jumanji: Welcome To The JungleDocument1 pageBukit Tinggi Booking Number: 990110 Jumanji: Welcome To The JungleNorzaifee NizamudinNo ratings yet
- Take5 OilnGasDocument8 pagesTake5 OilnGasNorzaifee NizamudinNo ratings yet
- Flue Gas Treatment For Co2 Capture - ccc169Document61 pagesFlue Gas Treatment For Co2 Capture - ccc169Norzaifee Nizamudin100% (1)
- The Financial Benefits of Cleaner Production (CP)Document11 pagesThe Financial Benefits of Cleaner Production (CP)Norzaifee NizamudinNo ratings yet
- Physical, Chemical and Biological Characteristics of WastewaterDocument45 pagesPhysical, Chemical and Biological Characteristics of WastewaterNorzaifee Nizamudin100% (2)
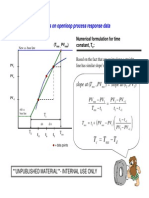
- Numerical Time ConstantDocument1 pageNumerical Time ConstantNorzaifee NizamudinNo ratings yet
- Carta Organisasi AblePerfectDocument1 pageCarta Organisasi AblePerfectNorzaifee NizamudinNo ratings yet
- Yoko Zeki 2008Document10 pagesYoko Zeki 2008Norzaifee NizamudinNo ratings yet
- Guideline NadopodDocument28 pagesGuideline NadopodNorzaifee NizamudinNo ratings yet
- Hero Found: The Greatest POW Escape of the Vietnam WarFrom EverandHero Found: The Greatest POW Escape of the Vietnam WarRating: 4 out of 5 stars4/5 (19)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureFrom EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureRating: 5 out of 5 stars5/5 (125)
- The End of Craving: Recovering the Lost Wisdom of Eating WellFrom EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellRating: 4.5 out of 5 stars4.5/5 (82)
- Sully: The Untold Story Behind the Miracle on the HudsonFrom EverandSully: The Untold Story Behind the Miracle on the HudsonRating: 4 out of 5 stars4/5 (103)
- The Fabric of Civilization: How Textiles Made the WorldFrom EverandThe Fabric of Civilization: How Textiles Made the WorldRating: 4.5 out of 5 stars4.5/5 (58)
- Transformed: Moving to the Product Operating ModelFrom EverandTransformed: Moving to the Product Operating ModelRating: 4 out of 5 stars4/5 (1)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestFrom EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestRating: 4 out of 5 stars4/5 (28)
- Mini Farming: Self-Sufficiency on 1/4 AcreFrom EverandMini Farming: Self-Sufficiency on 1/4 AcreRating: 4 out of 5 stars4/5 (76)
- The Future of Geography: How the Competition in Space Will Change Our WorldFrom EverandThe Future of Geography: How the Competition in Space Will Change Our WorldRating: 4 out of 5 stars4/5 (6)
- When the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachFrom EverandWhen the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachRating: 3.5 out of 5 stars3.5/5 (6)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaFrom EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNo ratings yet
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindFrom EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo ratings yet
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyFrom EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyNo ratings yet
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationFrom EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationRating: 4.5 out of 5 stars4.5/5 (46)
- Pale Blue Dot: A Vision of the Human Future in SpaceFrom EverandPale Blue Dot: A Vision of the Human Future in SpaceRating: 4.5 out of 5 stars4.5/5 (588)
- Four Battlegrounds: Power in the Age of Artificial IntelligenceFrom EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceRating: 5 out of 5 stars5/5 (5)
- Permaculture for the Rest of Us: Abundant Living on Less than an AcreFrom EverandPermaculture for the Rest of Us: Abundant Living on Less than an AcreRating: 4.5 out of 5 stars4.5/5 (33)
- The Book of the Moon: A Guide to Our Closest NeighborFrom EverandThe Book of the Moon: A Guide to Our Closest NeighborRating: 4.5 out of 5 stars4.5/5 (11)
- Restoration Agriculture: Real-World Permaculture for FarmersFrom EverandRestoration Agriculture: Real-World Permaculture for FarmersRating: 4.5 out of 5 stars4.5/5 (86)
- Project Management All-in-One For DummiesFrom EverandProject Management All-in-One For DummiesRating: 5 out of 5 stars5/5 (6)
- Reality+: Virtual Worlds and the Problems of PhilosophyFrom EverandReality+: Virtual Worlds and the Problems of PhilosophyRating: 4 out of 5 stars4/5 (24)
- Process Plant Equipment: Operation, Control, and ReliabilityFrom EverandProcess Plant Equipment: Operation, Control, and ReliabilityRating: 5 out of 5 stars5/5 (1)