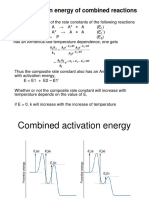
Professional Documents
Culture Documents
Chain Mech
Chain Mech
Uploaded by
Nabila Agnasia DesmaraCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chain Mech
Chain Mech
Uploaded by
Nabila Agnasia DesmaraCopyright:
Available Formats
Free Radical Chain Mechanism
k
1
1. Br
2
2 Br inititation
k
2
2. Br + H
2
HBr + H propagation
k
3
3. H + Br
2
HBr + Br propagation
k
4
4. H + HBr H
2
+ Br inhibition
k
5
5. 2 Br Br
2
breaking
experimental rate law:
d[HBr]
dt
=
k
a
[H
2
][Br
2
]
1
/
2
k
b
+k
c
[HBr]
[Br
2
]
I.
d[HBr]
dt
= k
2
[Br][H
2
] + k
3
[H][Br
2
] k
4
[H][HBr]
II.
d[Br]
dt
= 0 = 2 k
1
[Br
2
] k
2
[Br][H
2
] + k
3
[H][Br
2
] + k
4
[H][HBr] 2 k
5
[Br]
2
III.
d[H]
dt
= 0 = k
2
[Br][H
2
] k
3
[H][Br
2
] k
4
[H][HBr]
IV. add II + III : 0 = 2 k
1
[Br
2
] 2 k
5
[Br]
2
[Br] =
k
1
k
5
[Br
2
]
1
/
2
V. solve III for [H] : [H] =
k
2
[Br][H
2
]
k
3
[Br
2
]+k
4
[HBr]
=
k
2
k
1
k
5
1
/
2
[H
2
][Br
2
]
1
/
2
k
3
[Br
2
]+k
4
[HBr]
VI. subtract III from I:
d[HBr]
dt
= 2 k
3
[H][Br
2
]
substitute V into VI:
d[HBr]
dt
=
2k
3
k
2
k
1
1
/
2k
5
-1
/
2[H
2
][Br
2
]
1
/
2[Br
2
]
k
3
[Br
2
]+k
4
[HBr]
d[HBr]
dt
=
2k
3
k
2
k
1
1
/
2k
5
-1
/
2[H
2
][Br
2
]
1
/
2
k
3
+k
4
[HBr]
[Br
2
]
Explosions: 2 H
2
+ O
2
2 H
2
O
H
2
+ (O
2
) 2 OH initiation
H
2
+ OH H + H
2
O propagation
(O
2
) + H O + OH branching
O + H
2
OH + H branching
You might also like
- 레이먼드 창의 기본일반화학 14판 연습문제 답 (짝수 번호만 포함)Document4 pages레이먼드 창의 기본일반화학 14판 연습문제 답 (짝수 번호만 포함)키No ratings yet
- Practice FinalDocument7 pagesPractice FinalSamuel RobertsNo ratings yet
- Test Questions 2009Document69 pagesTest Questions 2009Dana CapbunNo ratings yet
- Chem Problem Solving .5 2235Document110 pagesChem Problem Solving .5 2235Book of Life fgfhfghfghfgh50% (4)
- CH4 2Document26 pagesCH4 2梁智翔No ratings yet
- KineticsDocument107 pagesKineticsK CabeguinNo ratings yet
- 26 The Kinetics of Complex Reactions: V K (CHDocument4 pages26 The Kinetics of Complex Reactions: V K (CHLukman HakimNo ratings yet
- C©u 1. Cho PH N Øng PH©N Huû Xiclobutan TH NH Etylen C: Etylen Xiclobu X X X X K K K KDocument2 pagesC©u 1. Cho PH N Øng PH©N Huû Xiclobutan TH NH Etylen C: Etylen Xiclobu X X X X K K K KNguyễn Khắc ToànNo ratings yet
- MechanismsDocument1 pageMechanismsVijay PradhanNo ratings yet
- Yesica Narváez 102113011313Document3 pagesYesica Narváez 102113011313yessi_narvaez5461No ratings yet
- 2 - Reaction Mechanisms For Non-Elementary ReactionsDocument24 pages2 - Reaction Mechanisms For Non-Elementary ReactionsFrederick JoseNo ratings yet
- The Activation Energy of Combined Reactions: e A e A e A K K KDocument16 pagesThe Activation Energy of Combined Reactions: e A e A e A K K KJAIME REDOLFO YUPANQUINo ratings yet
- K00337 - 20180906103219 - SKF3023 LECTURE 4aDocument33 pagesK00337 - 20180906103219 - SKF3023 LECTURE 4aAin SufizaNo ratings yet
- Turorial-1 - Cl302 Fogler Solution PDFDocument3 pagesTurorial-1 - Cl302 Fogler Solution PDFshubhamNo ratings yet
- Lec13 2012Document16 pagesLec13 2012Ervin CrespoNo ratings yet
- Week 06 SolutionDocument2 pagesWeek 06 SolutionbelenoteroalvaradoNo ratings yet
- F Solution INCHO 2010Document14 pagesF Solution INCHO 2010Towshif AliNo ratings yet
- Chemistry Class Xi Exe. ProblemsDocument227 pagesChemistry Class Xi Exe. ProblemsramchanderNo ratings yet
- UZEBIM - PHA 110 - Example QuestionsDocument9 pagesUZEBIM - PHA 110 - Example QuestionsAbba UmarNo ratings yet
- Chapter 10Document2 pagesChapter 10Namit KumarNo ratings yet
- Formacion de ComplejosDocument4 pagesFormacion de Complejosqlvanessa50No ratings yet
- Equations AnswersDocument6 pagesEquations AnswersGunjan KumarNo ratings yet
- Reaksi KationDocument9 pagesReaksi KationAnnisa AmaliaNo ratings yet
- Deriving Rate Laws Using The Steady-State Approximation - Part IDocument4 pagesDeriving Rate Laws Using The Steady-State Approximation - Part IGürkan KarakaşNo ratings yet
- Chap 3Document23 pagesChap 3Van Nguyen Phuong NganNo ratings yet
- Chapter 3 - Chain ReactDocument23 pagesChapter 3 - Chain ReactHien HoangNo ratings yet
- Bismuth ChlorideDocument4 pagesBismuth ChlorideChengkc2014No ratings yet
- ElectrodeDocument2 pagesElectrodeThatcher PanchoNo ratings yet
- HomogenDocument2 pagesHomogenNurul KhaerahNo ratings yet
- Homo GenDocument2 pagesHomo GenNurul KhaerahNo ratings yet
- Tutorial SolutionsDocument26 pagesTutorial SolutionsshubhamNo ratings yet
- UPM CHEM 18 Problem Set 1Document3 pagesUPM CHEM 18 Problem Set 1Vanessa ValdezNo ratings yet
- Vbtcoorlevel 0Document4 pagesVbtcoorlevel 0Alex SamNo ratings yet
- Chemical Reference Material: Formulae and Names of Some Common IonsDocument8 pagesChemical Reference Material: Formulae and Names of Some Common Ionsbmnyandu2003No ratings yet
- Arrangement of Coefficients in The Equations of Redox ReactionsDocument11 pagesArrangement of Coefficients in The Equations of Redox Reactionslorenlori2004No ratings yet
- 2008 Tadross2Document98 pages2008 Tadross2potpuraaaNo ratings yet
- Chemical Kinetics Cinetica ChimicaDocument42 pagesChemical Kinetics Cinetica Chimicalavinia diaNo ratings yet
- Tutorial 10 Alkanes - AnswersDocument7 pagesTutorial 10 Alkanes - AnswersEugene ChanNo ratings yet
- Multiple Choice QuestionsDocument39 pagesMultiple Choice QuestionsFatma JamalNo ratings yet
- Sumarni - Tugas Praktikum Modul 2Document3 pagesSumarni - Tugas Praktikum Modul 2SUMARNI 19TKM434No ratings yet
- Answer Booklet Sem 2 BOOK PDFDocument17 pagesAnswer Booklet Sem 2 BOOK PDFBryanLeeChienYungNo ratings yet
- CH 02Document17 pagesCH 02Simay OğuzkurtNo ratings yet
- Stoichiometry-Sheet: 2 (Balancing of Reactions) : Level - 1 1. 1. 2. 3. 4. 5. 6. 7Document2 pagesStoichiometry-Sheet: 2 (Balancing of Reactions) : Level - 1 1. 1. 2. 3. 4. 5. 6. 7Aarnav JainNo ratings yet
- CET DPP 14-8 - SolutionDocument2 pagesCET DPP 14-8 - SolutionNarayan BhatNo ratings yet
- The Dien Cuc ChuanDocument9 pagesThe Dien Cuc Chuanvinasat1108No ratings yet
- Experiments 3,4,5Document13 pagesExperiments 3,4,5Athirah JamalludinNo ratings yet
- Xii Chem Q and A CH 10Document18 pagesXii Chem Q and A CH 10SÁMÃÑ KANNANo ratings yet
- Chemical KineticsDocument18 pagesChemical KineticsRamchandra MurthyNo ratings yet
- Reaction MechanismDocument37 pagesReaction MechanismNurshuhada NordinNo ratings yet
- CPC Assignment 6Document5 pagesCPC Assignment 6Para DiseNo ratings yet
- Red Ox BalancingDocument2 pagesRed Ox BalancingAhmed KazazNo ratings yet
- BChO 2008 - SolutionsDocument8 pagesBChO 2008 - SolutionsPerfect SparkNo ratings yet
- Standard Reduction Potentials (At 25 C, 101.325 Kpa, 1M) Half-Reaction E (Volts)Document1 pageStandard Reduction Potentials (At 25 C, 101.325 Kpa, 1M) Half-Reaction E (Volts)sena_chem6706No ratings yet
- Soal Latihan Kuliah 1: Ra A RB B RR V RS SDocument5 pagesSoal Latihan Kuliah 1: Ra A RB B RR V RS SVidyanovaAnggunMentariNo ratings yet
- Obținerea Substanțelor Organice: 1. AlcaniDocument9 pagesObținerea Substanțelor Organice: 1. AlcaniAna-Maria GîlcaNo ratings yet
- Standard Reduction (Electrode) PotentialsDocument2 pagesStandard Reduction (Electrode) PotentialsdannyfunezNo ratings yet
- SUBSTITUTION REACTIONS by StevenDocument10 pagesSUBSTITUTION REACTIONS by StevendenderiNo ratings yet
- 27 MARCH 2020: Assignment 5 Question PaperDocument4 pages27 MARCH 2020: Assignment 5 Question PaperShadreck SandweNo ratings yet
- DU MaDocument18 pagesDU MaNova ChronoNo ratings yet
- Salt Analysis Cheat Sheet (Specially For New Era Public School)Document1 pageSalt Analysis Cheat Sheet (Specially For New Era Public School)Shubham GargNo ratings yet
- Chemsketch RomDocument24 pagesChemsketch RomDana CapbunNo ratings yet
- Chapter 15Document32 pagesChapter 15Dana CapbunNo ratings yet
- Chapter 14Document42 pagesChapter 14Dana CapbunNo ratings yet
- Sept 16Document6 pagesSept 16Dana CapbunNo ratings yet
- Chema:: Ancient Roots Ancient Roots IIDocument3 pagesChema:: Ancient Roots Ancient Roots IIDana CapbunNo ratings yet
- P13Document8 pagesP13Dana CapbunNo ratings yet
- P11 ADocument9 pagesP11 ADana CapbunNo ratings yet