Professional Documents
Culture Documents
Elements of Modern Style Article
Uploaded by
mamazookeeprCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Elements of Modern Style Article
Uploaded by
mamazookeeprCopyright:
Available Formats
Elements of [Modern] Style
Ytterby there are two secret pasts. Two
centuries ago, the sleepy village- now
dotted with vacation homes belonging
to wealthy residents of nearby
Stockholm- was a restless mining settlement, shipping
out high-grade feldspar for the royal porcelain
factories of Europe and quartz to line the blast
furnaces springing up across England. It is also the
birthplace of some of natures most wondrous and
least appreciated chemical elements.
The latter story began in 1787, when an
amateur geologist named Carl Arrhenius was visiting a
mine in Ytterby. He discovered an unusually heavy
black rock among the gray outcroppings and, being a
man of healthy scientific curiosity, sent a sample for
analysis to Johan Gadolin, a prominent chemist at the
Royal Academy of Turku in Finland. In 1794 Gadolin
concluded that the specimen contained an entirely
new element, later named yttrium. By 1879 chemists
had isolated six additional elements from the same
rock, bringing the grand total in the newly invented
periodic table to 70. Three of those elementsytterbium, erbium, and terbium- were simply given
additional variants on the name Ytterby, while the
other three were named holmium (for Stockholm),
scandium, and thulium (both from the Latin for
Scandanavia), in the nationalistic fashion then in favor.
After a long, lucrative run, the Ytterby quarry was
closed in 1933. In many ways, though, the towns
influence looms larger than ever. The elements
discovered there, known collectively as rare earths,
today form the backbone of the modern wired and
wireless world- even though you have probably never
heard of them.
th
The name rare earths made sense to the 19 century mind; rare because it seemed at first that they
came only from Scandanavia, and earths because they
occurred in an earthly oxide form from which it was
exceptionally hard to obtain the pure metal. Today it
is clear that the rare earths are hardly rare. The most
th
common of them, cerium, ranks 25 in abundance in
the earths crust, one place ahead of homely copper.
Yttrium is twice as abundant as lead; all of the rareearth metals (with the exception of radioactive
promethium) are more common than
by Hugh Aldersey-Williams
Six valuable rare earths, shown in powdered oxide
form. Neodumium, a key component of electronic
devices, is at front.
silver. The earths part is also misleading. These
elements are actually metals, and quite marvelous
ones at that. The warm glow of terbium is essential to
high-efficiency compact-fluorescent bulbs. Europium
is widely exploited to make vivid displays for laptop
computers and smart phones. Rare earths also pop up
in more unexpected places like baseball bats,
European currency, and night-vision goggles.
With their growing popularity comes new
value, and even political notoriety. Terbium and
europium recently overtook silver in price, reaching
$40 an ounce. The growing demand for rare earths
has become the subject of numerous government
reports and a bill that passed in the House of
Representatives. The reason these elements are
causing such a stir is not their scarcity but their
inaccessibility. Rare earths tend to occur in hard rock
such as granites, where they lump together in a
uniform way that makes them difficult to extract.
Separating out the desired elements
demands a toxic and dangerous process, and China
has the best infrastructure for doing so economically.
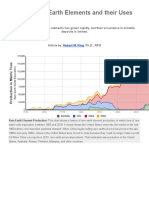
China holds about 36 percent of the worlds 110
million tons of recoverable rare-earth ores, with the
rest scattered worldwide, principally in the United
States, India, Australia, and Russia. Yet China currently
produces as much as 97 percent of the worlds rareearth oxides, according to the US Government
Accountability Office. Pekka Pyykk, a professor of
chemistry at the University of Helsinki, puts it this way:
Elements of [Modern] Style
by Hugh Aldersey-Williams
Not all the deposits are in China, but the processing
capacity right now is.
Supply would not matter if not for demand,
and the demand comes from the unusual electrical
properties of the rare earths- or lanthanides, as
chemists prefer to call them, because they mostly
follow lanthanum in the periodic table of elements.
The lanthanides share similar chemical properties
because they all react similarly, mostly with their three
outer electrons. (An atoms arrangement of electrons
is what determines most of its physical and chemical
attributes.) Like copper, iron, cobalt, and other more
familiar metals, lanthanides form many colored
compounds. The magic happens when those outer
electrons change energy states and release visible
light. But the rare earths are especially valuable for
their property of fluorescence: They can absorb light
or ultraviolet rays and re-emit the energy as an eerie
glow of certain colors specific to each element. The
brilliant emission of red and green is the reason why
lanthanides are indispensable components of todays
television sets and compact fluorescent bulbs.
From a technological perspective, a more
intriguing trait of the rare earths is that some of them
are highly magnetic. Alloyed with other metals, they
make extraordinarily strong and compact magnets:
perfect for computer hard drives, cordless power
tools, microphones, and headphones. An iPod takes a
triple sip of rare earths: to store digital music, to recreate it in earbuds, and to display what is playing. An
Life on Lanthanides: Toyota Prius
Toyota has sold 2 million Priuses globally since the cars
introduction in 1997, including a million in the United
States. Unfortunately, while the worlds most popular
hybrid sips gasoline, it devours rare earths. The Prius gets
its juice from a about 20 pounds of lanthanum, which
shuttles electrons between the batterys positive and
negative terminals. Powerful magnets in the Priuss electric
motors incorporate neodymium, whose cost shot up 150
percent in the first third of 2011. The price of lanthanum
has increased sevenfold, to $60 a pound, over the past year,
and in 2010 China briefly suspended rare-earth shipments to
Japan in a diplomatic dispute. Toyota is working with the
electric car company Tesla Motors to install rare-earth-free
motors in future vehicles.
iron alloy containing terbium and dysprosium has a
particularly useful property: It expands and contracts
efficiently in the presence of a magnetic field.
Sensors, actuators, and injectors commonly use such
materials, for instance to regulate the flow of gasoline
into an auto motive engine.
Compact, powerful magnets are key
components for efficient generators and electric
motors, and this makes rare earths star players in
green technology. (Molycorp Minerals- the biggest
rare-earth mining operation in the United Statesoptimistically calls them the green elements.)
Lanthanide-flecked supermagnets are used to
generate electricity in wind turbines. A turbine
capable of generating 2.5 megawatts of power
typically uses 700 pounds of neodymium. High-speed
maglev trains make extensive use of such magnets to
levitate the cars above their tracks. The Toyota Prius
is practically a rolling
exhibit of rare
earths, containing
eight different
elements, for a total
of 25 pounds of rare
earthiness.
Life on Lanthanides: Baseball Bats
Are you the weakest player on your company softball team?
One perfectly legal way to boost your performance is to use
a metal bat infused with the rare-earth element scandium.
When mixed with aluminum, the silvery-white element
shrinks and refines the grains of the metal, making an alloy
that is highly resistant to cracking. Just a little bit lets you
build a stronger bat without sacrificing resilience and durability, says Dewey Chauvin, director of bat engineering at
Easton Sports. You can add more features to the bat, like a
longer barrel and hence a larger sweet spot, without incurring weight. But scandium production is so low that the
element- which is also used in bicycle frames and lacrosse
sticks- can cost hundreds of dollars a pound, limiting its use
to top-of-the-line bats priced at $150 or more.
Elements of [Modern] Style
Life on Lanthanides: Smart Phones
The era of ever-shrinking consumer electronics would be
virtually impossible without rare earths. Neodymium is one
of the most important elements here because of its
powerful magnetic properties. In modern cell phones,
neodymium is a key ingredient in the wafer-thin permanent
magnet that converts electric signals to sound waves in
speakers and headsets. It is also used in the small motors
that vibrate when you turn your ringer off. Other rare
earths are crucial for the vivid displays in smart phones,
televisions, and laptops. Europium and terbium emit red
and green light, respectively, and their market value rose
more than 75 percent in the first four months of 2011.
Expect demand to sharpen as color touch screens become
more common with the spread of tablets and e-readers.
But the commercial versatility of
the lanthanides extends far beyond
the world of electronics. Cerium is
a prime example. It was discovered
in 1803 by Swedish chemist Jns Jacob Berzelius, who
named it after the asteroid Ceres, discovered two
years earlier. (Ceres was then regarded as a new
planet and so was much celebrated.) In 1885 the
Viennese chemist Carl Auer von Welsbach found that a
pinch of cerium oxide, together with thorium oxide,
usefully increases the brightness of a gas lamp. Soon
after, Auer discovered that a mixture of cerium and
iron readily generates sparks. The alloy, which he
modestly called Auermetall, is still used to ignite the
flame in cigarette lighters.
Ceriums applications kept multiplying. A
fine powder of cerium oxide, known as opticians
rouge, is an excellent abrasive for polishing glass.
Cerium compounds form nontoxic red pigments that
are used in toys and household products. In medicine,
cerium compounds were once administered for
everything from tubercular coughing to seasickness;
today those treatments are mostly regarded as
quackery, but the element still earns its place in
hospitals as an antiseptic wash for severe burns.
You may have cerium oxide in your kitchen if
your oven is a self-cleaning model; it helps break down
burned-on leftovers. You certainly have cerium oxide
in your car, assuming it is less than 35 years old. The
by Hugh Aldersey-Williams
compound works to convert carbon monoxide
emissions in automotive exhaust to less harmful
carbon dioxide. As a fine powder mixed into diesel
fuel, cerium oxide can also clean up the sooty fumes
produced by trucks and buses. Industry consumes
55,000 tons of cerium every year. The element, pulled
from a type of weathered granite called monazite,
fetches about $60 a pound.
Useful and yet so unfamiliar: the average
person is still largely in the dark about rare earths,
even though many of them are literally casting light
into the world. Cerium is a crucial element in the
intense, white-light arc lamps used by moviemakers to
illuminate the action on-set. Glass doped with cerium
oxide effectively absorbs ultraviolet light. That makes
it useful for UV-blocking windows and sunglasses, as
well as for protecting X-ray equipment from radiation
damage. Two other rare-earth metals, neodymium
and praseodymium, work their magic at the other end
of the spectrum. They impeded infrared light and so
are incorporated into welders goggles to protect
workers eyes from the heat. These oxides also absorb
yellow and green, so glass made with them has a
characteristic mauve tinge.
More broadly, rare earths add color to our
world because of their rich and varied optical
properties. The glazes on earthenware dishes and
vases commonly contain oxides of tin (white), copper
(blue), or iron (amber). But rare-earth oxides can
dissolve in the glaze to create more exotic hues, such
as pink and lime green, with a sharp, electric
appearance that artists love. They can be
transparent, which really brings ceramics alive, says
David Pier, a ceramicist in Chapel Hill, North Carolina.
Adding erbium oxide, for example, gives a subtle tone-
The Mountain Pass mine in California restarted
operations earlier this year, providing a rare-earth
alternative to China.
Elements of [Modern] Style
more pink lemonade than Pepto-Bismol, as Pier
describes it.
Colorful fluorescence is the calling card of
another rare-earth element with another odd name:
europium. Elements were frequently named for the
town or region where they were discovered,
sometimes in a very provincial manner, as was the
case with yttrium. Europium runs against the trend. It
was the last of the natural lanthanides to be found, in
1901. In that year, Frenchman Eugne-Anatole
Demaray isolated the element as an impurity in
samples of another rare earth, samarium (named,
indirectly, after Russian mine official V. E. SamarskyBykhovets). In a fit of magnanimity, Demaray
decided to name his element not after Paris, where he
worked, or after France, but in honor of all Europe.
all the rare earths, europium is perhaps
the most visible. It is used in the
phosphors of display screens, where it
emits red and blue light. A little europium
is also added to mercury vapor streetlamps to whiten
their otherwise cold blue light. It even has found its
way into the wallet. Officials at the European Central
Bank evidently decided it would be fitting if europiumbased compounds were added to euro banknotes as
fluorescent security markings. When the notes are
inspected under a banks ultraviolet scanner, pictures
of a bridge and the European continent light up in
green. The relationship between europium and the
euro was not announced; it was revealed only when
Freek Suijver and Andries Meijerink, two curious
chemists at the University of Utrecht in the
Netherlands, put the notes in their spectrometer.
They had a good laugh but were unable to determine
who concocted this sophisticated inside joke. I
learned that the use of europium was intentional,
Meijerink says, but I am sorry to say that I have no
inside information on who, where, and how it was
decided to use the europium luminescence.
The importance of the rare earths is no secret
in the industrialized world, however. One year ago,
when China announced it would limit exports, the
commodity price of all the rare earths almost instantly
shot up. In just six months the price of samarium
oxide quadrupled, to more than $50 a pound in May
by Hugh Aldersey-Williams
of this year, owing to its property as a permanent
supermagnet when alloyed with cobalt. Samariums
ability to stay strongly magnetic in extreme
temperatures makes it a favorite of the military for
use in precision-guided weapons. In April, China took
steps to keep prices high by extending a national ban
on rare-earth exploration and the opening of new
mines.
For all the dire headlines, the latest rareearth frenzy may be little more than a bubble. Not so
long ago, China aggressively pursued cheaper means
of isolating rare earths, benefiting from the absence of
costly environmental regulations, which is how the
country acquired its current near-monopoly. But its
dominance could well be temporary. Because of its
cheap prices, the competitors closed down, says
Pekka Pyykk. In the present situation, new capacity
will undoubtedly be restarted.
Mountain Pass, California, is one of the
places where American rare earths may rise again.
Here, Molycorp used to meet much of the worlds
demand for these elements. The company shuttered
its operations in 2002, but earlier this year it reopened
its mine. Another American company, U.S. Rare
Earths, owns mineral rights to lanthanide resources in
Montana and Idaho; according to a report by the
Government Accountability Office, these are in early
exploratory stages of development. Mines in these
areas may take up to 15 years to bring online, the
report states, largely due to the time it takes to
comply with multiple state and federal regulations.
A visit to other parts of the periodic table
could also turn up substitutes for some of the
lanthanides. In January, Toyota- spooked by rising
prices and supply concerns- announced that it is
developing a new type of propulsion, called an
induction motor, for future electric and hybrid
vehicles. The technology notably does not rely on
rare-earth elements.
For now, the world is hooked on the
lanthanides, and China is the only place that has the
processing capacity up and running. As for Ytterby,
that mine at least is unlikely to reopen. It is now a
historic landmark, located just off the intersection of
Terbium and Mine roads. A small sign commemorates
Elements of [Modern] Style
by Hugh Aldersey-Williams
it on the quayside, noting the sites prodigious
contribution to chemistry.
Life on Lanthanides: Wind Turbines
Carbon-free electricity gets a big boost from rare earths.
Behind the rotors of a wind turbine, wire coils move through
a magnetic field to generate current. To maximize
efficiency, manufacturers like General Electric often use
high-intensity magnets that incorporate rare-earth
elements. Some turbines contain 700 pounds of
neodymium. Without rare earths, youd have to use larger,
heavier magnets, and youd have to build a stronger, more
expensive tower to support them, says materials scientist
Alex King of the U.S. Department of Energys Ames
Laboratory. Last year GE received a $2 million federal grant
to develop magnets with only one-fifth the rare-earth
content of todays offerings.
You might also like
- Rare EarthDocument10 pagesRare Earthjagadish.kvNo ratings yet
- A Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksFrom EverandA Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksNo ratings yet
- HTTP WWW - Technologyreview.com Printer Friendly ArticleDocument3 pagesHTTP WWW - Technologyreview.com Printer Friendly ArticleAurelio SartoriNo ratings yet
- Copper EssayDocument5 pagesCopper EssayDouglay McTay0% (1)
- Written Report in GenChemistryDocument6 pagesWritten Report in GenChemistryAlexisNo ratings yet
- How Is Yttrium MadeDocument11 pagesHow Is Yttrium MadeBagas AdiansyahNo ratings yet
- Chapter 8 GPHDocument63 pagesChapter 8 GPHKevinNo ratings yet
- REE Rare EarthsDocument11 pagesREE Rare EarthsRicardo JesusNo ratings yet
- Planetary Metal&Herbal CorrespondencesDocument30 pagesPlanetary Metal&Herbal CorrespondencesCor ScorpiiNo ratings yet
- REE - Rare Earth Elements: Home MetalsDocument10 pagesREE - Rare Earth Elements: Home MetalsMorkizgaNo ratings yet
- Members: Saz, Frederico Toh, Marvin Unahan, Shierwin Jee Borba, Godfrey Ventero, Patrick Siacor, Breezy Mae EyleDocument44 pagesMembers: Saz, Frederico Toh, Marvin Unahan, Shierwin Jee Borba, Godfrey Ventero, Patrick Siacor, Breezy Mae EylemalynNo ratings yet
- Need To FinishDocument6 pagesNeed To FinishCely Marie VillarazaNo ratings yet
- Background of The StudyDocument18 pagesBackground of The StudyCely Marie VillarazaNo ratings yet
- Transition Metals II: (General Chemistry)Document11 pagesTransition Metals II: (General Chemistry)Erica Bianca MirandaNo ratings yet
- Chemistry ProjectDocument4 pagesChemistry ProjectThet HtarNo ratings yet
- Handbook of Extractive Metallurgy Copper: Per and of The Corresponding Words in MostDocument9 pagesHandbook of Extractive Metallurgy Copper: Per and of The Corresponding Words in Most1394203031No ratings yet
- Business of Rare Earth ElementsDocument6 pagesBusiness of Rare Earth ElementsAnanthNo ratings yet
- Stone-Age People Knew Nothing of Metal. Colorful: Hematite Iron Ore Were Found, They Would Be GroundDocument2 pagesStone-Age People Knew Nothing of Metal. Colorful: Hematite Iron Ore Were Found, They Would Be Groundlink2u_007No ratings yet
- Gold Iron Copper OxygenDocument3 pagesGold Iron Copper OxygenfranshuNo ratings yet
- Material WorldDocument188 pagesMaterial WorldYahaya HassanNo ratings yet
- Tungsten ItiaDocument24 pagesTungsten ItiaGottumukkala Venkateswara RaoNo ratings yet
- MS4SSA-Introduction To MetalsDocument62 pagesMS4SSA-Introduction To MetalsEdgar GadzikwaNo ratings yet
- Classification of MaterialsDocument113 pagesClassification of Materialsabhinavgiri17No ratings yet
- Byron Capital Markets Rare Earths Industry ReportDocument20 pagesByron Capital Markets Rare Earths Industry Reportgeorg1967No ratings yet
- CopperDocument35 pagesCopperjagan005No ratings yet
- General Chemistry Script and FlowDocument7 pagesGeneral Chemistry Script and FlowdecastroghislaneNo ratings yet
- 2.6.a-Reshaping Our LivesDocument14 pages2.6.a-Reshaping Our Livestwinkledreampoppies50% (2)
- Classification of MaterialsDocument113 pagesClassification of Materialsabhinavgiri17No ratings yet
- Characteristics of Iron OreDocument4 pagesCharacteristics of Iron OreNathan SwamiNo ratings yet
- Vasilyev Metals and ManDocument285 pagesVasilyev Metals and Manatto_11100% (1)
- Chemical Elements Crossword 1Document2 pagesChemical Elements Crossword 1David LockartNo ratings yet
- Group Jupiter 1Document21 pagesGroup Jupiter 1zin GuevarraNo ratings yet
- Invest in Transitional MetalsDocument12 pagesInvest in Transitional MetalsTristan WagnerNo ratings yet
- Invest in Transitional MetalsDocument12 pagesInvest in Transitional MetalsTristan WagnerNo ratings yet
- Unit 2B.1Document16 pagesUnit 2B.1PualeilehuaNo ratings yet
- 2.copper Latin Name:cuprum: 3.gold Latin Name:aurum Symbol:Au When:1848 Where:California Who:James Wilson MarshallDocument4 pages2.copper Latin Name:cuprum: 3.gold Latin Name:aurum Symbol:Au When:1848 Where:California Who:James Wilson MarshallPatricia B. LedesmaNo ratings yet
- Carbon Magic: by David Lincoln AUG 2018Document37 pagesCarbon Magic: by David Lincoln AUG 2018David LincolnNo ratings yet
- CopperDocument18 pagesCopperaimanhazimNo ratings yet
- 10 Copper Facts - Https://egyptiansorcery - ComDocument2 pages10 Copper Facts - Https://egyptiansorcery - ComAravenaNo ratings yet
- Iron and The EnvironmentDocument15 pagesIron and The EnvironmentIzzy ConceptsNo ratings yet
- A Three Day Fasting and PrayerDocument5 pagesA Three Day Fasting and PrayerBroadsageNo ratings yet
- MS4SSA IntroductiontoMetalsDocument62 pagesMS4SSA IntroductiontoMetalsSrikanth SrikantiNo ratings yet
- Chapter 2 RRLDocument31 pagesChapter 2 RRLIkamuzu OturanNo ratings yet
- The Importance of Iron in Our Daily LifeDocument38 pagesThe Importance of Iron in Our Daily Lifelamia97100% (1)
- 5 Electricity and Chemistry: ElectrolysisDocument12 pages5 Electricity and Chemistry: Electrolysistotatote034No ratings yet
- Nickel OccurrenceDocument16 pagesNickel OccurrenceArfinsa AinurzanaNo ratings yet
- IridiumDocument6 pagesIridiumCh V S RajuNo ratings yet
- Copper Design ManualDocument22 pagesCopper Design Manualamr_scorpion_engNo ratings yet
- METALSDocument62 pagesMETALSEstefany MarilagNo ratings yet
- History of MetalDocument5 pagesHistory of Metalrbnlth.joNo ratings yet
- Mars and IronDocument3 pagesMars and IronRimuel92No ratings yet
- Prospecting and Mining Basics of Gold and Base MetalsDocument93 pagesProspecting and Mining Basics of Gold and Base MetalsKaharuddin HawingNo ratings yet
- Metal UsesDocument8 pagesMetal UsesallogyNo ratings yet
- Ele City: (Investigatory Project)Document8 pagesEle City: (Investigatory Project)Liyana ChuaNo ratings yet
- Earth Science Quarter 1 - Module 2Document4 pagesEarth Science Quarter 1 - Module 2Shelly Ryn SaligumbaNo ratings yet
- Chemistry Midterm EssayDocument5 pagesChemistry Midterm EssayIrakli NatroshviliNo ratings yet
- Mining Obscure MetalsDocument3 pagesMining Obscure MetalsRXillusionistNo ratings yet
- Earth - S Natural Wealth - An AuditDocument6 pagesEarth - S Natural Wealth - An AuditBrando MezNo ratings yet
- Electrical CordsDocument34 pagesElectrical CordsShiela Mae BigataNo ratings yet
- Average Weighted Atomic MassDocument2 pagesAverage Weighted Atomic MassmamazookeeprNo ratings yet
- Hydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andDocument1 pageHydrate Lab: Never Place A Hot Crucible On A Balance! When Cool, Determine The Mass of The Crucible andmamazookeeprNo ratings yet
- Chemical Formula QuizDocument3 pagesChemical Formula QuizmamazookeeprNo ratings yet
- Unit 4 Test 2009Document2 pagesUnit 4 Test 2009mamazookeeprNo ratings yet
- Build A Trend LabDocument2 pagesBuild A Trend LabmamazookeeprNo ratings yet
- Subatomic Particle WorksheetDocument1 pageSubatomic Particle WorksheetmamazookeeprNo ratings yet
- Lab Report Format: Title Source Name Partner Date PurposeDocument2 pagesLab Report Format: Title Source Name Partner Date PurposemamazookeeprNo ratings yet
- PopcornDocument2 pagesPopcornmamazookeeprNo ratings yet
- Laboratory Exercise III: Significant FiguresDocument1 pageLaboratory Exercise III: Significant FiguresmamazookeeprNo ratings yet
- Laboratory Exercise IV: Density LabDocument2 pagesLaboratory Exercise IV: Density LabmamazookeeprNo ratings yet
- Sample Lab ReportDocument3 pagesSample Lab Reportmamazookeepr63% (8)
- Significant Figures Jokes 2Document2 pagesSignificant Figures Jokes 2mamazookeeprNo ratings yet
- Significant Figures Jokes 1Document2 pagesSignificant Figures Jokes 1mamazookeeprNo ratings yet
- B9 Main Ideas ML-1Document3 pagesB9 Main Ideas ML-1rd4tp5k25gNo ratings yet
- Lesson Plan On Body Parts For EslDocument2 pagesLesson Plan On Body Parts For Eslapi-265046009No ratings yet
- Books Published in Goa 2007Document24 pagesBooks Published in Goa 2007Frederick NoronhaNo ratings yet
- ResumeDocument4 pagesResumeapi-233282347No ratings yet
- AMLTM.00.092-00-Iss1 Tape Adhesion Test For Paints and CoatingsDocument6 pagesAMLTM.00.092-00-Iss1 Tape Adhesion Test For Paints and CoatingsalbertoNo ratings yet
- IPC ProjectDocument15 pagesIPC ProjectAshish ChandaheNo ratings yet
- Comparative Analysis of Print Media and Digital MediaDocument32 pagesComparative Analysis of Print Media and Digital MediaDeval Jyoti ShahNo ratings yet
- Escala TimpDocument8 pagesEscala TimpLucy GonzalesNo ratings yet
- The List, Stack, and Queue Adts Abstract Data Type (Adt)Document17 pagesThe List, Stack, and Queue Adts Abstract Data Type (Adt)Jennelyn SusonNo ratings yet
- Group 14 Monitoring SystemDocument27 pagesGroup 14 Monitoring SystemAndrei 26No ratings yet
- Acc 103 Course OutlineDocument3 pagesAcc 103 Course OutlineAnitha KimamboNo ratings yet
- Natural Disasters That Frequently Occur in The PhilippinesDocument30 pagesNatural Disasters That Frequently Occur in The PhilippinesTokemi MoariNo ratings yet
- BBC-Cellpack Produktbroschuere Smart Sensors EN 0821Document12 pagesBBC-Cellpack Produktbroschuere Smart Sensors EN 0821Javier CuzcoNo ratings yet
- Applied Geography: Marco VizzariDocument11 pagesApplied Geography: Marco Vizzarialexandra mp100% (1)
- QMS Assignment 1Document3 pagesQMS Assignment 1John Michael PadillaNo ratings yet
- Q4 HEALTH 9 MODULE 3 WEEK 3 and 4.BetaTestedDocument22 pagesQ4 HEALTH 9 MODULE 3 WEEK 3 and 4.BetaTestedCyris Gay AdolfoNo ratings yet
- TABLE OF SPECIFICATIONS - Grade-10 (4th Grading)Document4 pagesTABLE OF SPECIFICATIONS - Grade-10 (4th Grading)Aljon Jay Sulit SiadanNo ratings yet
- DAFZA Security - User ManualDocument28 pagesDAFZA Security - User Manualzubeen RIZVINo ratings yet
- 03.2 ENSTP - 2019 Aggregates PART1Document24 pages03.2 ENSTP - 2019 Aggregates PART1VEHERNo ratings yet
- Clinker CoolersDocument76 pagesClinker CoolersShariq Khan100% (6)
- Tructured Uery Anguage: 1 ©stefan Stanczyk P00482 - 2005Document71 pagesTructured Uery Anguage: 1 ©stefan Stanczyk P00482 - 2005Sky ShetuNo ratings yet
- Contemporary WorldDocument2 pagesContemporary WorldPrince LitchNo ratings yet
- Ground Floor PlanDocument1 pageGround Floor PlanManu MannuNo ratings yet
- Faraki Cross-Domain Similarity Learning For Face Recognition in Unseen Domains CVPR 2021 PaperDocument10 pagesFaraki Cross-Domain Similarity Learning For Face Recognition in Unseen Domains CVPR 2021 PaperData LOG NGUYENNo ratings yet
- Soliton WavesDocument42 pagesSoliton WavesAbdalmoedAlaiashyNo ratings yet
- Victorias Climate Science Report 2019Document48 pagesVictorias Climate Science Report 2019Alexander DarlingNo ratings yet
- References For Motivation and IncentivesDocument2 pagesReferences For Motivation and Incentiveskitson200100% (1)
- 28.3.2022 - Practice Test - SsDocument3 pages28.3.2022 - Practice Test - SsTran Hanh DanNo ratings yet
- Extemporaneous RubricDocument2 pagesExtemporaneous RubricGeorge Kevin TomasNo ratings yet
- LIGHT - MIRROR Practice QuestionsDocument2 pagesLIGHT - MIRROR Practice Questionsshrish000000100% (1)