Professional Documents
Culture Documents
Synthesis, Characterization of New Schiff Base and Some Metal Complexes Derived From Glyoxylic Acid and O-Phenylenediamine
Uploaded by
Andzhiita SaampeerOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Synthesis, Characterization of New Schiff Base and Some Metal Complexes Derived From Glyoxylic Acid and O-Phenylenediamine
Uploaded by
Andzhiita SaampeerCopyright:
Available Formats
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
Synthesis, Characterization Of New Schiff Base And Some
Metal Complexes Derived From Glyoxylic Acid And
O-Phenylenediamine
J. Sh. Sultan
Department of Chemistry,
University of Baghdad
Email: ja.sultan@yahoo.com.
College
of
Education,
Ibn-Al-Haitham,
Received in : 27 May 2012 Accepted in : 7 August 2012
Abstract
The new Schiff base, namely (2-Amino-phenylimino)-acetic acid (L) was prepared
from condensation of glyoxylic acid with o-phenylene diamine. The structure (L) was
characterized by, IR, 1H, 13C-NMR and CHN analysis. Metal complexes of the ligand (L)
were synthesized and their structures were characterized by Atomic absorption, IR and UVVisible spectra, molar conductivity, magnetic moment and molar ratio determination (Co+2,
Cd+2) complexes. All complexes showed octahedral geometries.
Key words: Synthesis, Characterization, Schiff base, glyoxylic acid, O-phenylenediamine
and metal ions
Introduction
Glyoxilic acid and its derivatives play important roles in natural processes,
participating in glyoxylate cycle which functions in plants and in some microorganism[1-4].
The presence of aldehyde in the glyoxilic acid allows numerous a cyclic derivatives
containing C=N bond- azomethines and hydrazones[5-8].
The aim of this work is to synthesize and study the coordination behaviour of the new
ligand (2-Amino-phenylimino)-acetic acid (L) and its complexes with Co+2, Ni+2, Cu+2 , Cd+2,
Hg+2 and Pb+2.
Experimental
All chemicals were purchased from BDH, and used without further purifications.
Instrumentation
1. FTIR spectra were recorded in KBr on Shimadzu- 8300 Spectrophotometer in the range of
(4000-400 cm1).
2. The electronic spectra in H2O were recorded using the UV-Visible spectrophotometer type
(spectra 190-900 nm) CECIL, England, with quartz cell of (1 cm) path length.
3. The melting point was recorded on "Gallen kamp Melting point Apparatus".
4. The Conductance Measurements were recorded on W. T. W. conductivity Meter.
5. Metal analysis. The metal contents of the complexes were determined by atomic absorption
(A. A.) technique. Using a shimadzu PR-5. ORAPHIC PRINTER atomic obsorption
spectrophotometer.
Chemistry - 264
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
6. Balance Magnetic Susceptibility model MSB-MLI Al-Nahrain University
7. The characterize of new ligand (L) is achieved by:
A: 1H and 13C-NMR spectra were recorded by using a bruker 300 MHZ (Switzerland).
Chemical Shift of all 1H and 13C-NMR spectra were recorded in (ppm) unit
downfield from internal reference tetramethylsilane (TMS), using D2O as a solvent.
B: Elemental analysis for carbon, hydrogen was using a Euro Vector EA 3000 A Elemental
Analysis (Italy).
C: These analysis (A and B) were done in at AL-al-Bayt University, Al- Mafrag, Jordan.
Synthesis
1. Synthesis of (2-Amino-phenylimino)-acetic acid (L)
To a hot solution of O-phenylenediamine (0.074g. 1m mole) in (5ml) of ethanol, a hot
solution of glyoxylic acid (0.108 g. 1 m mole) in (5ml) of ethanol was added. The solution
was refluxed for 3.5 hrs. Upon cooling a dark brown precipitate formed, was filtered off and
recrystallized from a hot mixture of [(5ml) methanol, (5ml) acetone and (2ml) distilled water].
A dark brown precipitate, yield 85%, melting point 98- 100C, CHN, C= 58.53 (58.51),
H = 4.87 (4.69).
2. Synthesis of complexes
The complex LCoCl.2H2O has been synthesized as follows:
To a hot solution of ligand (L) (0.164g. 1m mole) in (5ml) of ethanol, a hot solution of
cobalt(II) Chloride. hexa hydrate (0.238g. 1m mole) in (5 ml) of ethanol was added. The
precipitate immediately formed, the mixture was boiled and stirring for 10-15 min., filtered
off. Recrestallized from a hot of (10ml) methanol, a dark green precipitate, yield 80%,
decomposed at 110 D.
The physical properties for synthesized ligand (L) and its complexes are shown in
Table (1).
A similar method was used to prepare other complexes: LNiCl.2H2O, L(0.164g 1m
mole), NiCl2.6H2O (0.238g, 1m mole), (10ml) ethanol, (10ml) methanol, yield 90%
decomposed at 200 D, LCuCl.2H2O, L(0.164g, lm mole), CuCl2.2H2O (0.170g, 1m mole),
(10ml) ethanol, (10ml) methanol, yield 82% decomposed at 180 D, LCdCl.2H2O, L(0.164g,
lm mole), CdCl2 .H2O (0.202g, 1m mole), (10ml) ethanol, (10ml) methanol, yield 95%
decomposed at 160 D, LHgCl.2H2O, L(0.164g, lm mole), HgCl2 (0.271g, 1m mole), (10ml)
ethanol, (10ml) methanol, yield 78% decomposed at 190 D, LPb(NO3) 2.2H2O, L(0.164g, lm
mole), Pb(NO3)2 (0.331g, 1m mole), (10ml) ethanol, (10ml) methanol yield 82% decomposed
at 210 D.
IR spectrum of the ligand (L)
The IR spectrum of the (L) Fig. (1) shows new strong bands at (1737, 1668) cm1 are
due to (C=O) of carboxylic group and HC=N imine[8-9] compared with the precursors Figs.
(23), Table (4), which indicate the ligand (L) has been obtained.
Bands corresponding to CH aromatic stretching at (3061) cm1[1,5], NH 2 at (3385,
3363) cm1 are observed[1,3].
Absorption occurs as a sharp peak in the 3466 cm1 is attributed to free (unassociated)
hydroxylCH2 COOH group[5,7,9].
Chemistry - 265
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
UV- spectrum of the ligand (L)
The UV- spectrum of (L) Fig. (4), Table (5) was recorded in distilled water with the
range (210 400) nm. The molar absorption at (261) nm may be assigned to an *
transition[10].
NMR spectrum for the ligand (L)
1
H- NMR spectrum of the ligand (L) in DMSOd6 Table (2), Fig. (5) is characterized
by the appearance of chemical shift related to the NH2 protons- aromatic NH2 at 5.10 ppm.,
Chemical shift of aromatic protons showed at 6.777.539 ppm. The characteristic signals at
8.21 ppm. is assigned to HC=N. The COOH signal is found at 10.354 ppm.
13
CNMR of the free ligand Table (3), Fig. (6) shows the HC=N peak at 143.50 ppm.,
the COOH peak at 170 ppm. and carbon peaks for aromatic are detected at 110-125
ppm.[5,11,12].
The IR spectra for the complexes
The free ligand exhibits a strong absorption band at (1737) cm1 due to the stretching
vibration of (C=O) of the carboxylic group. This band is disappeared in the spectra of its
complexes accompanied by the appearance of two bands one in the (1569-1514)cm1 range
due to asymm. (COO) and another bands in the (1398-1375) cm-1 range assigned to symm.
(COO), = (171-139) cm-1 . Fig. (7), Table (4). This indicates that the carboxylic group is
monodentate coordinate[13-14].
The appearance of stretching modes assigned to NH2 and HC=N of C=NH groups
was observed at (33753456) cm-1 and (16601620) cm-1 respectively in free ligand[1-3].
The stretching vibration of azomethine group of the ligand was shifted to lower
frequencies in all spectra, whereas stretching vibrations of NH2 getting broad indicating
additional coordination of metal ions to NH2 and (C=N) group[1-5].
Bands related to coordinate water were observed in all spectra; Cd+2 (704) cm-1, Ni+2
(713) cm-1, Hg+2 (621) cm-1 , Cu+2 (669) cm-1, Co+2 (775) cm-1 and Pb+2 (806) cm-1. Additional
bands were observed at lower frequencies (600400) cm-1 and were attributed to MN, MO
stretching modes[1-2,5,8].
Lead complex shows band at 1660 cm1 due to the NO 3 group.
The electronic absorption spectral and magnetic studies
The Co(II) complex exhibited band around (490) nm (20408) cm-1 (max=300 molar1
cm1) Table (5), which was assigned to 4T1g(F) 4T1 g(P), for high spin octahedral geometry.
The magnetic susceptibility measurements (4.50) BM Table (1), for the solid Co(II)
complex is indicated of three unpaired electrons per Co(II) ion consistent with its odctahedral
environment[9,16-17].
The electronic absorption spectrum of the Ni(II) complex showed broad band center at
(460)nm (21739cm1) (max=400molar1 cm1) assigned to the spin-allowed transition
3
A2g(F)3T1g(P) consistent with octahedral configuration[19,22]. The magnetic moment (2.90) BM
suggested two unpaired electrons per Ni(II) also consistent with octahedral geometry. The electronic
absorption spectrum of Cu(II) complex Fig. (8) showed broad band at (800) nm (12500) cm1
(max=224 molar1 cm1), which was assigned to 2Eg2T2g transition, typical for an octahedral
Chemistry - 266
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
configuration. The magnetic moment (1.80) BM suggested one unpaired electron for Cu(II)
consistent with its octahedral environment[18-19].
The spectra of Cd+2, Pb+2 and Hg+2 complexes exhibited charge transfer bands, which
were assigned to a ligand to metal charge transfer[12,20].
Solutions chemistry
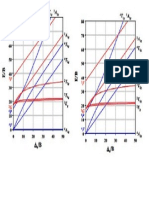
Molar ratio
The complexes of the ligand (L) with selected ions (Co+2, Cd+2) were studied in
solution using water as solvents, in order to determine (M:L) ratio in the prepared complexes,
following molar ratio method[21].
A series of solutions were prepared having a constant concentration (C) 103 M of the
hydrated metal salts and the ligand (L). The (M:L) ratio was determined from the relationship
between the absorption of the observed light and mole ratio (M:L) found to be (1:1). The
result of complexes formation in solution are shown in Table (69), Fig. (910).
To determined G[15]:
k = ML/[M][L]
(1)
= (Am As)/ Am
(2)
k = The equation (1) is written to mole ratio (1:1) as the following
(3)
kf = (1 )/ 2 C
= max.b.c.
(4)
kf = stability constant
= decomposition Degree
M = metal ion
L = The ligand
[ ] = concentration
As = The absorption of the equivalent point of mole ratio
Am = The maximum absorption of the mole ratio
C = The complex concentration (mole. L1).
G = - 2.303 RT Log K.
R = 8.303
T = 273 + 25 = 298
Molar conductivity for the complexes of the ligand (L)
The molar conductance of the complexes in water Table (10) lies in the (3.40 0.61) S.
cm2 molar1 range, indicating their non electrolyte nature, except for the Cu complex which
its molar conductance lies in the (119) S. cm2 molar1 range, indicating its electrolytic nature
with (1:1) ratio[22].
Chemistry - 267
Ibn Al-Haitham Journal for Pure and Applied Science
No.
25
Vol.
2012
2012
Year
25
Conclusion
The Schiff base ligand (L) is prepared and charcterised by C, H, N and 1H, 13CNMR.
The ligand (L) is behaved as tridentate mode: NH2, CH=N and O forming octahedral
below:
complexes with M+2, where M+2 = Co, Ni, Cu, Cd, Hg and Pb SchemeCO
O
H2N
OH
OH
NH2
H
H2N
NH2
+ HX
M
H2O
H2O
X2
C
O
M = Co+2, Ni+2, Cu+2, Cd+2, Hg+2 and Pb+2
X = Cl in all complexes except in lead complex
X = ONO2
References
1.
2.
3.
4.
5.
6.
7.
8.
Mishchenco, A. V.; Lukov, V. V. and Popov, L. D. (2011) synthesis and physicochemical study of complexation of glyoxylic acid arolhydrazone, with Cu(II) in solution
and solid phale, Joural of coordination chemistry, 64(11):1963- 1976.
Arif, M.; Qurashi, M. M. R. and Shad, M. A. (2001) metal- based antibacterial agents;
synthesis, characterization, and in vitro biological evaluation of cefixime- derived
Schiff bases and their complexes with Zn(II), Cu(II), Ni(II) and Co(II), Journal of
coordination chemistry, 64(11):1914-1930.
Dominik, C. and Branko, K. (2011) schiff base derived from 2-hydroxyl-1naphthaldehyde and liquid- assisted mechanochemical synthesis of its isostructural
Cu(II) and Co(II) complexes, crystengcomm, 13: 4351-4357.
Abdulghani, A. J. and Abbas, N. M. (2011) synthesis characterization and biological
Activity study of New Schiff and mannich bases and some metal complexes derived
from isatin and dithiooxamide, Hindawi publishing corporation bioinorganic chemistry
and applications, p. 1-15.
Cemal, S.; Zeliha, H. and Hakan, D. (2011) synthesis, characterizations and structure of
NO doner Schiff base ligands and nickel(II) and copper(II) complexes, Journal of
Molecular structure, 977: 53- 59.
Anant, P. and Singh, K. K. (2011) synthesis, spectroscopy and biological studies of
Nickel(II) complexes with tetradentate shicff basce having N2O2 donor group, J. Dev.
Biol. Tissue. Engineering, 3 (2):13-19.
Kamellia, N. and Razie, S. (2011) synthesis and mesomorphic of symmetric
tetradentate shicff bases based on azo-containing salicylaldimines and their copper (II)
complexes, Journal of coordination chemistry, 64(11): 1859-1870.
Sajjad, M.; Shokoh, B. and Asad, Sh. (2011) Hetero trinuclear manganese (II) and
Vanadium (IV) Schiff base complexes, as epoxidation catalysts, transition met chem..,
36: 425-431.
Chemistry - 268
Ibn Al-Haitham Journal for Pure and Applied Science
No.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
Vol.
25
Year
2012
2012
25
Raj, K. D. and Sharad, K. M. (2011) synthesis, spectroscopic and antimicrobial studies
of new iron (III) complexes, containing Schiff bases and substituted benzoxazole
ligands, Journal of coordination chemistry, 64(13):2292-2301.
Fleming, I. and William, D. H. (1966) "Spectroscopic methods in organic chemistry",
Ed. McGraw Hill publishing company ltd, London.
Tajmir, R. (1990) coordination chemistry of vitamin c. part I. Interaction of L-Ascorbic
Acid with Alkaline Earth Metal Ions in the Crystalline Solid and Aqueous Solution, J.
Inorg. Biochem, 40:181-188.
Tajmir, R. (1991) Coordination chemistry of vitamin C. part (II). Interaction of LAscorbic Acid with Zn(II), cd(II), Hg(II), and Mn(II) Ions in the solid state and in
Aqueous solution, Int. J. Inorg. Biochem, 42: 47-55.
Geeta, B. and Ravinder, V. (2011) synthesis, characterization and biological evaluation
of mononuclear Co(II)m Ni(II) and Pd(II) complexes, with New N2O2 schiff base
ligand, chem.., pharm. Bull., 95 (2):166-171.
Washed, M. G.; Refat, M. S. and Megharbel, S. M. (2009) "Synthesis spectroscopic and
thermal characterization of some transition metal complexes of folic acid",
spectrochimia acta A, 70(4): 916922.
Sutton, D. (1968) Electronic spectra of Transition Metal complexes Mc GRAW-HILL.,
London.
Malcolm, J. A.; Gordonk, A. and Nigam, P. R. (1999) Synthesis and characterization of
platinum (II) complexes of L- Ascorbic Acid, Inorg. Chem., 38:5864-5869.
Orgel, L. (1966) "An Introduction to transition metal Chemistry", 2nd ed, Wiley, New
York.
Rakesh, K. Sh.; Munirathnam, N. and Ashoka, G. S. (2008) Asymmetric allylic
alkylation by palladium- bisphosphinites, Tetrahedron; Asymmetry, 19:555663.
Lever, P. A. B. (1968) "In organic electronic spectroscopy", Elsevier publishing
company, New York, 6:121.
Choi, K. Y.; Jeon, Y. M.; Lee, K. C.; Ryu, H.; Suh, M.; Park, H. S.; Kim, M. J. and
Song, Y. H. (2004) Preparation and characterization of a bidentate carboxylate bridged
dinuclear cadimium(II) complex with bis(2-pyridyl methyl) amino-3-propionic acid,
Journal of Chemical Crystollography, 34:591-596.
Skoog, D. A. and Donald, M. (1974) Fundamentals of Analytical chemistry Altoit
London Edition.
Kettle, S. F. (1975) "Coordination Compounds", Thomas Nelson and Sons, London,
P. 165.
Chemistry - 269
Ibn Al-Haitham Journal for Pure and Applied Science
No.
25
Vol.
2012
2012
Year
25
Table (1): The physical properties for synthesized lignad (L) and its complexes
Empirical
formula
M.P. C
Colour
85
98-100C
Dark brown
LCoCl.2H2O
80
110 D
Dark green
4.50
LNiCl.2H2O
90
200 D
Brown
2.90
LCuCl.2H2O
82
180 D
Dark brown
1.80
LCdCl.2H2O
95
160 D
brown
LHgCl.2H2O
78
190 D
pale brown
LPbNO3.2H2O
82
210 D
brown
L=C8H8O2N2,
NH2
5.10 ppm.
DMF = dimethyl formamide,
Found
(Calc.) %
metal
-
effect
Yield %
Solubility
Watar, methanol,
ethanol, ether, DMF,
DMSO
Watar, methanol,
ethanol, DMF, DMSO
=
(20.00)
19.66
(20.00)
19.31
(21.07)
21.80
(32.18)
32.45
(45.93)
45.46
(44.13)
44.50
=
=
=
=
DMSO = dimethyl sulfoxide,
D = Decomposition
Table (2): 1H-NMR Chemical shifts for L (ppm in D2O)
Aromatic proton
HC=N
COOH
6.77-7.539
8.221 ppm
10.354 ppm
Table (3): 13C-NMR Chemical shifts for L (ppm in D2O)
HC=N
COOH
Aromatic carbons
143.50 ppm.
170 ppm
110-125 ppm.
Table (4): Infrared spectral data (wave number ) cm1 for the ligand (L), precursors
and its complexes
Compound
Glyoxylic acid
O-phenylene
diamine
L
(OH)
(C-H)
Aromatic
3057
assm.
COO
-
1668
3061
1640
1624
1660
1614
1653
1622
1640
1620
1650
1620
1650
1630
3080
1558
1516
1560
1520
1565
1825
1550
1514
1541
1516
1569
1520
1398
1380
1396
1380
1394
1385
1386
1375
1398
1375
1384
1375
170
136
164
140
171
140
164
139
143
141
185
145
775
594
468
572
430
549
457
599
424
580
420
580
450
(C=O) (NH2) (C=N)
3361
-
1745
-
3466
1737
LCoCl.2H2O
LNiCl.2H2O
LCuCl.2H2O
LCdCl.2H2O
LHgCl.2H2O
LPbNO3.2H2O
3387
3363
3385
3363
3408
3375
3404
3375
3448
3376
3456
3379
3450
3380
3450
3376
3060
3134
3080
3064
3065
Chemistry - 270
symm. cm1 Coordinate
water
COO
-
713
669
704
621
806
MN
MO
-
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
Table (5): Electronic spectral data of the ligand (L) and its
wave number (max molar1
Compound
nm
cm1
cm1)
L
261
38314
325
LCoCl.2H2O
490
20408
300
LNiCl.2H2O
460
21739
400
LCuCl.2H2O
800
12500
224
LCdCl.2H2O
267
37453
463
LHgCl.2H2O
268
37313
398
LPbNO3.2H2O
268
37313
398
Where L=C8H8O2N2,
C.T.= Charge Transfer
VM
1 ml
1
1
1
1
1
1
1
1
1
25
metal complexes
Assignments
*
4
T1g(F)4T1 g(P)
4
A2 g(F)4T1g(P)
2
Eg2T2g
C. T.
C. T.
C. T.
Table (6): VM, VL and Absorption of ligand (L),
VM = volume of metal in ml, VL= volume of ligand in ml
[LCoCl.2H2O]
[LCdCl.2H2O]
VL
Abs
VM
VL
0.25
0.772
1 ml
0.25
0.50
1.251
1
0.50
0.75
1.624
1
0.75
1.00
1.950
1
1.00
1.25
2.050
1
1.25
1.50
2.174
1
1.50
1.75
2.289
1
1.75
2.00
2.404
1
2.00
2.25
2.500
1
2.25
2.50
2.601
1
2.50
Proposed
structure
Octahedral
=
=
=
=
=
Abs
0.783
1.092
1.455
1.755
1.964
2.084
2.250
2.403
2.558
2.695
Table (7): The absorbance values against mole ratio values of complex [LCoCl.2H2O]
in solution (1103 mole. L 1) in water at (272.8) nm
No.
L: M
absorbance
1
0.5:1
1.251
2
1:1
1.950
3
2:1
2.404
Table (8): The absorbance values against mole- ratio values of complex [LCdCl.2H2O]
in solution (1103 mole. L 1) in water at 272.8 nm
No.
L: M
absorbance
1
0.5:1
1.092
2
1:1
1.775
3
2:1
2.403
Table (9): Stability constant and G for the ligand (L) complexes
Compounds
As
Am
K
Log K
G
9
[LCoCl.2H2O]
1.950
2.404
0.19
2210
10.43
58.9
[LCdCl.2H2O]
1.755
2.403
0.27
1104
4
22.7
[LCoCl.2H2O] > [LCdCl.2H2O]
Chemistry - 271
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
Table (10): The molar conductance of the complexes*
Compound fragment ions
m S.cm2 molar1
LCoCl.2H2O
1.71
LNiCl.2H2O
0.61
LCuCl.2H2O
119
LCdCl.2H2O
1.5
LHgCl.2H2O
3.40
LPbNO3.2H2O
0.71
* Recorded in (water) solvent, where L=C8H8O2N2
Fig. (1): The IR spectrum of the ligand (L)
Fig. (2): The IR spectrum of glyoxylic acid
Fig. (3): The IR spectrum of O-phenylene diamine
Chemistry - 272
ratio
Neutral
Neutral
1:1
Neutral
Neutral
Neutral
Ibn Al-Haitham Journal for Pure and Applied Science
No.
Vol.
25
Year
2012
2012
25
Fig. (4): Electronic spectrum of the ligand (L)
Fig. (5): The 1H-NMR spectrum of the ligand (L)
Fig. (6): The 13C-NMR spectrum of the ligand (L)
Chemistry - 273
Ibn Al-Haitham Journal for Pure and Applied Science
25
Vol.
2012
2012
Year
25
Fig. (7): The IR spectrum of the (NiCl.2H2O) complex
Fig. (8): Electronic spectrum of the (CuCl.2H2O) complex
3.0
2.5
2.0
Absorbtion
1.5
1.0
0.5
0.0
0.0
0.5
1.0
1.5
2.0
2.5
Mole Ratio
Fig. (9): The mole ratio curve of complex [CoCl.2H2O] in solution (110-3 mole.
l-1) at (=272.8 nm)
3.0
2.0
Absorbtion
No.
1.0
0.0
0.0
0.5
1.0
1.5
2.0
2.5
Mole Ratio
Fig. (10): The mole ratio curve of complex [CdCl.2H2O] in solution (110-3 mole.
l-1) at (=272.8 nm)
Chemistry - 274
25
Ibn Al-Haitham Journal for Pure and Applied Science
2012
2012
Year
25
Vol.
No.
27: 2012 7: 2012
- 2) - ( - )(L
-
1H, 13CNMR ).(C.H.N
) (L
.
.
: -
.
Chemistry - 275
You might also like
- Coii Niii Cuii and Criii Complexes of Heterocyclic Schiff Base Ligand Synthesis Spectroscopic and Thermal StudyDocument5 pagesCoii Niii Cuii and Criii Complexes of Heterocyclic Schiff Base Ligand Synthesis Spectroscopic and Thermal StudyIJARP Publications100% (1)
- Organometallic Chemistry: Plenary Lectures Presented at the Eighth International Conference on Organometallic Chemistry, Kyoto, Japan, 12-16 September 1977From EverandOrganometallic Chemistry: Plenary Lectures Presented at the Eighth International Conference on Organometallic Chemistry, Kyoto, Japan, 12-16 September 1977Y. IshiiNo ratings yet
- Carborane Synthesis MethodsDocument17 pagesCarborane Synthesis MethodsJulienne Stephanie Fabie100% (1)
- ATOICV1 5 0 Isopoly and Heteropoly Acids and SaltsDocument46 pagesATOICV1 5 0 Isopoly and Heteropoly Acids and SaltsGokul KannanNo ratings yet
- Preparation and Characterization of Cobalt ComplexesDocument7 pagesPreparation and Characterization of Cobalt ComplexesIftitah HauriyahNo ratings yet
- Operation Manual: SpectrophotometerDocument21 pagesOperation Manual: SpectrophotometerMd shoriful islam100% (2)
- 1H NMR Spectrum Provides Structural InformationDocument53 pages1H NMR Spectrum Provides Structural InformationJian Hong Tee100% (1)
- Isopoly and Heteropoly Acids and SaltsDocument39 pagesIsopoly and Heteropoly Acids and SaltsAhilya GuptaNo ratings yet
- Metallic Carbonyls and NitrilesDocument40 pagesMetallic Carbonyls and NitrilesSandipan Saha100% (1)
- 12 Chemistry Ncert Ch09 Coordination Compounds Part 01 QuesDocument43 pages12 Chemistry Ncert Ch09 Coordination Compounds Part 01 Queshumayun khalidNo ratings yet
- Unit - 1 Lesson - 1Document271 pagesUnit - 1 Lesson - 1Rakesh SharmaNo ratings yet
- 1 IntroductoryDocument45 pages1 IntroductoryTuhin Sahu100% (1)
- 4.1.1 Protic Vs Aprotic SolventDocument36 pages4.1.1 Protic Vs Aprotic SolventDawit BirhanuNo ratings yet
- Solid State-1Document31 pagesSolid State-1ChirAgNo ratings yet
- Preparation of Metal CorbonylsDocument6 pagesPreparation of Metal Corbonylsyaqoob008No ratings yet
- Synthesis of NanomaterialsDocument34 pagesSynthesis of NanomaterialsDathan100% (2)
- Reactive Intermediates - LecturesDocument24 pagesReactive Intermediates - Lecturesapi-3771395100% (1)
- Carboranes: ClassificationDocument10 pagesCarboranes: ClassificationKrishna BhatiNo ratings yet
- Transition Metal ToxicityFrom EverandTransition Metal ToxicityG. W. RichterNo ratings yet
- Inorganic Chemistry D-Block ElementsDocument19 pagesInorganic Chemistry D-Block ElementsshinyeeNo ratings yet
- Vollhardt 6e Lecture PowerPoints - Chapter 11Document58 pagesVollhardt 6e Lecture PowerPoints - Chapter 11superfr3shmNo ratings yet
- Isomerism in Coordination Compounds PDFDocument7 pagesIsomerism in Coordination Compounds PDFmf720383270100% (1)
- 01 1350977450 79497 PDFDocument83 pages01 1350977450 79497 PDFArya ChowdhuryNo ratings yet
- D AND F BLOCK ELEMENT NotesDocument5 pagesD AND F BLOCK ELEMENT NotesM AroNo ratings yet
- Isolobal AnalogyDocument11 pagesIsolobal AnalogyGA GANo ratings yet
- Coordination Compound: IIT-JEE 2013Document50 pagesCoordination Compound: IIT-JEE 2013Utkarsh Agarwal100% (1)
- Notes Chapter 8 Transition ChemistryDocument17 pagesNotes Chapter 8 Transition ChemistryGauravRajNo ratings yet
- Umpolung reactivity: methods for interchanging carbonyl donor and acceptor reactivityDocument28 pagesUmpolung reactivity: methods for interchanging carbonyl donor and acceptor reactivitymeauna100% (1)
- SCH 206-Carboxylic Acids PDFDocument48 pagesSCH 206-Carboxylic Acids PDFShivani DamorNo ratings yet
- Chemical Bonding - HybridizationDocument3 pagesChemical Bonding - HybridizationVarsha YadavNo ratings yet
- Pearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesDocument5 pagesPearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesThantea ChhakchhuakNo ratings yet
- Free Radicals &carbocationsDocument13 pagesFree Radicals &carbocationsOmkar Kumar JhaNo ratings yet
- Classification of Organometallic CompoundsDocument28 pagesClassification of Organometallic CompoundsDingetegna GodanaNo ratings yet
- Complete Chpter#4 (The Periodic Table)Document8 pagesComplete Chpter#4 (The Periodic Table)shahshujaat75% (4)
- Polytechnic TRB Syllabus of ChemistryDocument4 pagesPolytechnic TRB Syllabus of ChemistrysanjeevNo ratings yet
- Resonance and Inductive Effects in Organic ChemistryDocument36 pagesResonance and Inductive Effects in Organic Chemistryeagl33yeNo ratings yet
- Hypervalent Iodine: Dess-Martin Periodane: Selective Oxidation of Prim. Alcohols To Aldehydes, Sec. Alcohols To KetonesDocument15 pagesHypervalent Iodine: Dess-Martin Periodane: Selective Oxidation of Prim. Alcohols To Aldehydes, Sec. Alcohols To Ketonesevsgoud_goudNo ratings yet
- PDFDocument155 pagesPDFHifza shairwani100% (1)
- Aromaticity PDFDocument9 pagesAromaticity PDFabyssabhi100% (2)
- Transition Metals and Coordination ChemistryDocument80 pagesTransition Metals and Coordination ChemistryVincent Choo100% (1)
- Ap SSC 10TH Class Physics BitsDocument10 pagesAp SSC 10TH Class Physics Bitsvenky97970% (1)
- Isomerism - Handwritten NotesDocument7 pagesIsomerism - Handwritten Notesgovind_galamNo ratings yet
- Reactive Intermediates: Arynes, Carbenes, and NitrenesDocument115 pagesReactive Intermediates: Arynes, Carbenes, and NitrenesMuhammad ArsalanNo ratings yet
- Quantitative and QualitativeDocument15 pagesQuantitative and QualitativesquadralsupremeNo ratings yet
- Synthetic Applications of Carbenes and NitrenesDocument13 pagesSynthetic Applications of Carbenes and NitrenesAnonymous g7uPednINo ratings yet
- Salt Analysis (Theory) - EngDocument28 pagesSalt Analysis (Theory) - Engjoxis70026100% (1)
- Structure of Atom: Rutherford's Nuclear ModelDocument12 pagesStructure of Atom: Rutherford's Nuclear ModelGautham GrimaceNo ratings yet
- Solid State PDFDocument35 pagesSolid State PDFAniruddha KawadeNo ratings yet
- Organic - Reagents FinalDocument27 pagesOrganic - Reagents FinalSankar AdhikariNo ratings yet
- Importance of C-C Cross Coupling Reactions in Pharmaceutical ChemistryDocument67 pagesImportance of C-C Cross Coupling Reactions in Pharmaceutical ChemistryAnonymous vRpzQ2BLNo ratings yet
- Magnetism Notes CompleteDocument11 pagesMagnetism Notes CompleteSathya Sai Kumar Yeluri100% (1)
- Chapter 3 Coordination ChemistryDocument41 pagesChapter 3 Coordination Chemistrytarun ratnaNo ratings yet
- D and f Block Elements PropertiesDocument8 pagesD and f Block Elements Propertiessrivathson7No ratings yet
- Mole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsDocument38 pagesMole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsR S.NagiNo ratings yet
- 2023-24 Coordination CompoundsDocument36 pages2023-24 Coordination Compoundsthe Skulptor100% (1)
- Practice Set 1Document1 pagePractice Set 1Lalit Ranjan SahuNo ratings yet
- INORGANIC CHEMISTRY EXPERIMENTSDocument46 pagesINORGANIC CHEMISTRY EXPERIMENTSpc355chyi100% (3)
- Lecture 1 OrganometallicsDocument19 pagesLecture 1 OrganometallicsAaron GrettonNo ratings yet
- FluorecenciaDocument7 pagesFluorecenciaAndzhiita SaampeerNo ratings yet
- 1 s2.0 S1878535210000791 MainDocument3 pages1 s2.0 S1878535210000791 MainAndzhiita SaampeerNo ratings yet
- Expo InglesDocument1 pageExpo InglesAndzhiita SaampeerNo ratings yet
- 098 - NiDMSO29 - 017 (1) Articulo Cientifico Quimica CoordinacionDocument2 pages098 - NiDMSO29 - 017 (1) Articulo Cientifico Quimica CoordinacionAndzhiita SaampeerNo ratings yet
- Quimica AmbientalDocument12 pagesQuimica AmbientalAndzhiita SaampeerNo ratings yet
- Tanabe 3dDocument1 pageTanabe 3dAndzhiita SaampeerNo ratings yet
- Protein AsDocument1 pageProtein AsAndzhiita SaampeerNo ratings yet
- Oralexam 2015 ADocument3 pagesOralexam 2015 AAndzhiita SaampeerNo ratings yet
- Determination of Some Transition Metals by Atomic Absorption Spectroscopy After Extraction With Pyridine-2-Aldehyde-2-QuinolylhydrazoneDocument2 pagesDetermination of Some Transition Metals by Atomic Absorption Spectroscopy After Extraction With Pyridine-2-Aldehyde-2-QuinolylhydrazoneAndzhiita SaampeerNo ratings yet
- Taller I - Simetría e Introducción CoordinaciónDocument3 pagesTaller I - Simetría e Introducción CoordinaciónAndzhiita SaampeerNo ratings yet
- J Cell Biol 2013 Antunes 365 79Document15 pagesJ Cell Biol 2013 Antunes 365 79Andzhiita SaampeerNo ratings yet
- NABARD road inspection report formatDocument24 pagesNABARD road inspection report formatSrinivas PNo ratings yet
- Kathy Davis - Dancing Tango - Passionate Encounters in A Globalizing World-New York University Press (2015)Document236 pagesKathy Davis - Dancing Tango - Passionate Encounters in A Globalizing World-New York University Press (2015)Csongor KicsiNo ratings yet
- GS16 Gas Valve: With On-Board DriverDocument4 pagesGS16 Gas Valve: With On-Board DriverProcurement PardisanNo ratings yet
- 2010 HD Part Cat. LBBDocument466 pages2010 HD Part Cat. LBBBuddy ButlerNo ratings yet
- Checklist of Requirements For OIC-EW Licensure ExamDocument2 pagesChecklist of Requirements For OIC-EW Licensure Examjonesalvarezcastro60% (5)
- Paradigms of ManagementDocument2 pagesParadigms of ManagementLaura TicoiuNo ratings yet
- Reading and Writing Q1 - M13Document13 pagesReading and Writing Q1 - M13Joshua Lander Soquita Cadayona100% (1)
- Iphoneos 31Document159 pagesIphoneos 31Ivan VeBoNo ratings yet
- CFO TagsDocument95 pagesCFO Tagssatyagodfather0% (1)
- Form Active Structure TypesDocument5 pagesForm Active Structure TypesShivanshu singh100% (1)
- Peran Dan Tugas Receptionist Pada Pt. Serim Indonesia: Disadur Oleh: Dra. Nani Nuraini Sarah MsiDocument19 pagesPeran Dan Tugas Receptionist Pada Pt. Serim Indonesia: Disadur Oleh: Dra. Nani Nuraini Sarah MsiCynthia HtbNo ratings yet
- Maharashtra Auto Permit Winner ListDocument148 pagesMaharashtra Auto Permit Winner ListSadik Shaikh50% (2)
- Annual Plan 1st GradeDocument3 pagesAnnual Plan 1st GradeNataliaMarinucciNo ratings yet
- WindSonic GPA Manual Issue 20Document31 pagesWindSonic GPA Manual Issue 20stuartNo ratings yet
- Dermatology Study Guide 2023-IvDocument7 pagesDermatology Study Guide 2023-IvUnknown ManNo ratings yet
- Technical Specification of Heat Pumps ElectroluxDocument9 pagesTechnical Specification of Heat Pumps ElectroluxAnonymous LDJnXeNo ratings yet
- Shouldice Hospital Ltd.Document5 pagesShouldice Hospital Ltd.Martín Gómez CortésNo ratings yet
- Statistical Decision AnalysisDocument3 pagesStatistical Decision AnalysisTewfic SeidNo ratings yet
- Technical specifications for JR3 multi-axis force-torque sensor modelsDocument1 pageTechnical specifications for JR3 multi-axis force-torque sensor modelsSAN JUAN BAUTISTANo ratings yet
- Technical Manual - C&C08 Digital Switching System Chapter 2 OverviewDocument19 pagesTechnical Manual - C&C08 Digital Switching System Chapter 2 OverviewSamuel100% (2)
- Steps To Christ AW November 2016 Page Spreaad PDFDocument2 pagesSteps To Christ AW November 2016 Page Spreaad PDFHampson MalekanoNo ratings yet
- Duca Industries March 2023 pay slip for Dipankar MondalDocument1 pageDuca Industries March 2023 pay slip for Dipankar MondalPritam GoswamiNo ratings yet
- 4 Influencing Factors of Learners Career Choice Parents Choice Vs Personal DescisionDocument24 pages4 Influencing Factors of Learners Career Choice Parents Choice Vs Personal Descisionmatteo mamaloNo ratings yet
- Exercise-01: JEE-PhysicsDocument52 pagesExercise-01: JEE-Physicsjk rNo ratings yet
- Public Private HEM Status AsOn2May2019 4 09pmDocument24 pagesPublic Private HEM Status AsOn2May2019 4 09pmVaibhav MahobiyaNo ratings yet
- Production of Sodium Chlorite PDFDocument13 pagesProduction of Sodium Chlorite PDFangelofgloryNo ratings yet
- Mpu 2312Document15 pagesMpu 2312Sherly TanNo ratings yet
- Returnable Goods Register: STR/4/005 Issue 1 Page1Of1Document1 pageReturnable Goods Register: STR/4/005 Issue 1 Page1Of1Zohaib QasimNo ratings yet
- Ovr IbDocument27 pagesOvr IbAriel CaresNo ratings yet
- Pasadena Nursery Roses Inventory ReportDocument2 pagesPasadena Nursery Roses Inventory ReportHeng SrunNo ratings yet