Professional Documents
Culture Documents
Hydrogen Sulfide Odor Control in Wastewater Collection Systems
Hydrogen Sulfide Odor Control in Wastewater Collection Systems
Uploaded by
Joe VergheseOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hydrogen Sulfide Odor Control in Wastewater Collection Systems
Hydrogen Sulfide Odor Control in Wastewater Collection Systems
Uploaded by
Joe VergheseCopyright:
Available Formats
57
HYDROGEN SULFIDE ODOR CONTROL
IN WASTEWATER COLLECTION SYSTEMS
BY PETER CHURCHILL* and DAVID ELMER**
[PRESENTED AT NEWEA ANNUAL CONFERENCE, JANUARY 1999]
INTRODUCTION
For some time, the Town of Bedford, Massachusetts had been receiving complaints about odors coming from the sewer system in neighborhoods downstream of
its Middlesex Turnpike Pumping Station. The odor complaints initiated an investigation of the sanitary collection system upstream of the neighborhoods. The layout
of the collection system indicated two potential problem areas. The Middlesex
Turnpike Pumping Station pumped sewage through a 2,500-foot force main, which
eventually fed a 21-inch reinforced concrete pipe with a very shallow slope. Both of
these areas had the potential to produce hydrogen sulfide (H2S); thus the Town decided to undertake an H2S monitoring program to further assess the problem.
PROJECT BACKGROUND
The Middlesex Turnpike Pumping Station collects domestic wastewater from
5,300 linear feet of gravity sewer. A substantial portion of the stations flow is generated during normal work hours (8am to 5pm, Monday through Friday). The service
area of the station is populated with numerous office parks and one major hotel/conference center with substantial dining facilities. Most of the office parks also have
on-site cafeterias for their staff, which contribute to the organic load. Biochemical
oxygen demand (BOD) in this area is strong, ranging from 300 to 320 milligrams per
liter (mg/l). Soluble BOD ranges from 71 to 150 mg/l.
As previously mentioned, the Middlesex Turnpike Pumping Station has a 2,500foot, 8-inch force main that carries flow to a gravity sewer on Crosby Drive, and has
detention times as long as 90 minutes. The gravity sewer on Crosby Drive feeds a
21-inch reinforced concrete pipe with a minimal slope on Route 62. The slow moving flow in this pipe creates another area for sulfide production. At the intersection
of Route 62 and Hemlock Lane, there is a manhole with a 4-foot hydraulic drop,
which also allows for the release of H2S.
ATMOSPHERIC HYDROGEN SULFIDE MONITORING
The Town used the Massachusetts Water Resources Authority (MWRA)
Community Assistance Program to install atmospheric H2S meters in three locations
downstream of the Middlesex Turnpike Pumping Station suspected of having the
highest H2S concentrations. The force main discharge manhole on Crosby Drive, the
manhole at the intersection of Crosby Drive and Route 62, and the drop manhole at
* Superintendent, Water and Sewer Dept., Town of Bedford, MA and ** Engineer, Weston & Sampson
Engineers, Inc.
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
58
HYDROGEN SULFIDE ODOR CONTROL
the intersection of Route 62 and Hemlock Lane were chosen as the locations for
Meters 1, 2 and 3, respectively. Each of these locations, shown in Figure 1, had turbulent flow conditions conducive to the release of sulfides to the atmosphere. Peak
atmospheric H2S readings recorded at these sites during the monitoring program were
81 parts per million (ppm), 36 ppm, and 80 ppm, respectively. H2S levels of these
magnitudes cause odor problems, pose health risks to collection system operators,
and may deteriorate the structural integrity of sewer infrastructure.
MIDDLESEX TURNPIKE
PUMP STATION
H2S
METER #4
H2S
METER #1
PRIVATE
PUMP STATION
PRIVATE
PUMP STATION
MEADOWBROOK
PUMP STATION
MILL DAM
PUMP STATION
H2S
METER #2
H2S
METER #3
DROP
MANHOLE
LEDGEWOOD
PUMP STATION
PAGE ROAD
PUMP STATION
Figure 1. LOCATIONS OF METERS INSTALLED
FOR H2S MONITORING PROGRAM
The results of the monitoring program showed that corrective measures needed to be
taken to eliminate residential complaints and protect the sewer system and worker
health and safety.
As a first attempt to resolve the problem, the Town used 25 percent sodium hydroxide to shock the force main to arrest H2S production. This technique successfully
decreased the atmospheric H2S concentrations at Meter 1 by killing sulfate-reducing
bacteria in the force main; however, the effect of the sodium hydroxide was shortlived, and concentrations quickly returned to previous levels, as Figure 2 shows. This
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
PETER CHURCHILL and DAVID ELMER
59
Figure 2. SODIUM HYDROXIDE DOSING
collection system would require a longer lasting, more-effective method of controlling sulfides.
H2S PRODUCTION
To effectively treat H2S in wastewater collection systems, one must understand the
mechanisms of its production. In collection systems, H2S is produced when bacteria
consume sulfate oxygen for organic processes. Sulfate-reducing bacteria grow in a
slime layer that coats the sewers wetted perimeter. These bacteria use oxygen in
the most readily available form: first, from elemental oxygen; then, nitrate oxygen;
then, sulfate oxygen. As nitrate is usually not available in wastewater, bacteria will
consume sulfate oxygen after depleting elemental oxygen, leaving bi-sulfide ions to
combine with hydrogen to form aqueous H2S. Figure 3 illustrates these reactions. At
pH 7, the bi-sulfide ion and aqueous H2S, in solution, are equally proportionate. pH,
Henrys Law, and the turbulence of the waste stream govern the rate at which aqueous H2S is converted to atmospheric H2S. A lower pH produces more aqueous H2S
and increases the rate of H2S transfer to the gas phase. Turbulent wastewater also
facilitates the release of H2S to the atmosphere.
As H2S is released into the sewer atmosphere, it combines with water on the crown
of the pipe to form sulfuric acid (H2SO4). Figure 3 also shows this reaction. Because
H2SO4 corrodes the sewer infrastructure, it is necessary to stop H2S production in the
collection system.
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
60
HYDROGEN SULFIDE ODOR CONTROL
Figure 3. HYDROGEN SULFIDE CHEMISTRY
PROJECT APPROACH
Manipulating the environment that bacteria use to support their growth is one way
to control sulfide odors. Our project approach, which involved bench-scale tests followed by a pilot study, sought to alter the environment conducive to H2S production
by introducing nitrate to the sewer system. Bacteria need oxygen to metabolize
organic matter. As was previously discussed, after consuming elemental oxygen,
bacteria will first use available nitrate, then sulfate. During our investigation, we
found that the dissolved oxygen concentration in the Middlesex Station wet well
averaged 4.4 mg/l. This aerobic wastewater was pumped through the force main each
time the pumping station was activated. The dissolved oxygen level in the wastewater decreased as the flow was pumped through the force main. After the elemental oxygen was consumed, the bacteria began to use alternate sources of oxygen.
Before the pilot program was implemented, that source of oxygen was in the form of
sulfate. Theoretically, the introduction of nitrate into the collection system should
provide an oxygen source for the bacterias use that will stop the reduction of sulfate,
which is the first step in sulfide production.
We contacted an agricultural supply house and found that there were several
nitrate-based chemicals that could be purchased. Table 1 shows a list of nitrate chemicals commonly available. Calcium nitrate was selected because of its low price and
ease of handling.
Table 1. NITRATE PRODUCTS
Product
Cost per Ton
Cost per Pound
Nitrate of Soda
Potassium Nitrate
Calcium Nitrate
$366
$646
$312
$0.183
$0.323
$0.156
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
61
PETER CHURCHILL and DAVID ELMER
Bench Tests
We conducted a series of bench tests to evaluate the nitrate concentrations of various mixtures of calcium nitrate and water. By adjusting the number of pounds of dry
chemical added to the water in the chemical feed tank, we were able to increase or
decrease the nitrate level to a desired concentration. Table 2 presents the cost of calcium nitrate at various concentrations.
Table 2. CALCIUM NITRATE BENCH TESTS
CaNO3
(grams/200ml))
Lab Concentration
Nitrate (mg/l)
Pounds of CaNO3
(needed for 1 gallon)
Cost of CaNO3
($/lb)
0
10
20
50
297*
34,000
60,000
140,000
440,000
0.417
0.834
2.086
12.391
$0.15
$0.15
$0.15
$0.15
$0.15
Cost of CaNO3
($/gallon)
$0.06
$0.13
$0.31
$1.86
* Grams of calcium nitrate at saturation
Pilot Program
Our pilot study included the following basic elements:
A chemical pump (LMI) to regulate the gallons of chemical discharged each day.
A 376-gallon plastic chemical feed tank.
A 1/4-hp mixer to dissolve the dry product.
A metal shed to house the equipment.
The Town of Bedford Department of Public Works staff performed all the work to
wire the station for power and provide water.
To monitor the pilot programs results, we installed four atmospheric H2S meters
at the following locations: the Middlesex Turnpike Pumping Station; the force main
discharge manhole on Crosby Drive; the manhole at the intersection of Crosby Drive
and Route 62; and the drop manhole at the intersection of Route 62 and Hemlock
Lane. Throughout the pilot study, these monitors took readings in five-minute increments. Grab samples were also collected from the monitoring sites to supplement the
atmospheric data. Two grab samples were taken at each of the monitoring locations
to establish baseline data, and additional samples were taken each time the calcium
nitrate concentration or feed rate was adjusted.
Two factors controlled the amount of nitrate oxygen being introduced to the wet
well: the concentration of the calcium nitrate solution and feed rate of the pump. The
chemical concentration varied between 140,000 and 350,000 mg/l of nitrate. A concentration of 140,000 mg/l of nitrate was found to be the easiest to administer and the
most cost-effective for controlling odors. The chemical feed rate varied from 35 to
67 gallons per day (gpd). The best results were obtained when the feed rate was
between 45 and 60 gpd. According to Table 2, about 622 pounds of calcium nitrate
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
62
HYDROGEN SULFIDE ODOR CONTROL
dissolved in 298 gallons of water was required to discharge 45 gpd of solution with
a nitrate concentration of 140,000 mg/l. This chemical solution cost about $93 to
make and had a duration of 6.5 days.
RESULTS
Two points during the pilot program clearly illustrated the effectiveness of the
treatment system. The first occurred at the beginning of the program when the chemical feed system was turned on. During the 11 days before the system was started up,
atmospheric H2S levels were recorded as high as 68 ppm at the force main discharge
manhole. On May 11, 1998, the pilot tank was turned on and the atmospheric level
decreased to zero ppm over the next 3 days, as Figure 4 shows.
Figure 4. CHEMICAL FEED START
The H2S monitors recorded levels less than 6 ppm through June 23, when they
were removed. (Monitors were also removed from June 3 to June 9). The second
illustration of the programs effectiveness occurred on August 14. The atmospheric
monitors were reinstalled on August 11, but the pilot tank was not restarted until
August 14. Figure 5 shows that the atmospheric H2S readings prior to pilot tank operation were as high as 34 ppm at the force main discharge manhole, and then decreased
to zero three days after the chemical feed tank was restarted.
Calcium nitrate was less effective in reducing atmospheric H2S concentrations further downstream at the intersection of Crosby Drive and Route 62 and at the Hemlock
Lane drop manhole; however, each location experienced a noticeable decrease in H2S
concentrations. Peak readings decreased from 36 to 25 ppm at Crosby Drive and
Route 62, and from 80 to 32 ppm at Hemlock Lane. An additional chemical feed
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
PETER CHURCHILL and DAVID ELMER
63
Figure 5. CHEMICAL FEED RESTART
station could be installed at Crosby Drive and Route 62 to control the release of sulfides in this section of the collection system.
CONCLUSIONS
There are many approaches to deal with H2S odors. One broad approach is to
change the environment within which the bacteria live. We chose this alternative by
introducing nitrate, which bacteria will seek out as an oxygen source before using sulfate, breaking the sulfide production chain. Depending on the configuration of individual collection systems, multiple dosing stations may be required to accomplish
this task.
Nitrate addition provides an easy, cost-effective way to control H2S odors in collection systems. Bedfords pilot program cost the Town under $1,000 to implement
and effectively eliminated odor complaints, arrested the corrosion of the sewer
infrastructure, and provided a safer work environment for collection system operators.
REFERENCES
1. Metcalf and Eddy, Inc., Wastewater Engineering Treatment, Disposal, and
Reuse (Third Edition), McGraw Hill, Inc., New York, 1991.
2. Sawyer, Clair N., and McCarty, Perry L., Chemistry For Sanitary Engineers
(Second Edition), McGraw Hill Book Co., 1967.
NEWEA JOURNAL, MAY 1999
VOL. 33 NO. 1
You might also like
- Metrode WPS SuperduplexDocument4 pagesMetrode WPS SuperduplexClaudia Mms100% (3)
- Temper Bead Welding ProceduresDocument8 pagesTemper Bead Welding ProceduresClaudia Mms100% (1)
- Welding of Stainless Steels and Other Joining Methods: A Designers' Handbook Series N 9 002Document18 pagesWelding of Stainless Steels and Other Joining Methods: A Designers' Handbook Series N 9 002Sreenivas GuduruNo ratings yet
- Odor and Corrosion Control in Sanitary Sewerage Systems and Treatment PlantsDocument142 pagesOdor and Corrosion Control in Sanitary Sewerage Systems and Treatment PlantsLuis Antonio OrtegaNo ratings yet
- Environmental Engineering NotesDocument8 pagesEnvironmental Engineering NotesI am UnstoppableNo ratings yet
- Static Equipment in Oil and Gas IndustryDocument93 pagesStatic Equipment in Oil and Gas IndustryRaghavanNo ratings yet
- Water ChemistryDocument483 pagesWater ChemistryYasin ERKOC100% (1)
- Characterization and Control of Odours and VOC in the Process IndustriesFrom EverandCharacterization and Control of Odours and VOC in the Process IndustriesNo ratings yet
- Odour ControlDocument4 pagesOdour ControlkumarNo ratings yet
- Inspection and Welding Repairs of Pressure VesselsDocument9 pagesInspection and Welding Repairs of Pressure VesselsClaudia Mms100% (1)
- Owen Barfield BibliographyDocument50 pagesOwen Barfield BibliographyClaudia MmsNo ratings yet
- Design of Biofilter OdorDocument9 pagesDesign of Biofilter OdorNicolás SepúlvedaNo ratings yet
- Odor LawsDocument27 pagesOdor LawsΔημητρηςΣαρακυρουNo ratings yet
- Odour PreventionDocument416 pagesOdour PreventionSaber Abdel MoreidNo ratings yet
- Odour Control in WWTPDocument10 pagesOdour Control in WWTPMadhavan DurairajNo ratings yet
- Pickling HandbookDocument20 pagesPickling HandbookRhona100% (1)
- Odor Threshold Determinations of 53 Odorant ChemicalsDocument6 pagesOdor Threshold Determinations of 53 Odorant ChemicalsT. LimNo ratings yet
- Odor ControlDocument9 pagesOdor ControlSabrina Dorado VinsonNo ratings yet
- Activated Sludge ModellingDocument16 pagesActivated Sludge ModellingJeremy Dudley100% (1)
- Certification Study Guide For Water Treatment Operators: The Training Station ©2002Document0 pagesCertification Study Guide For Water Treatment Operators: The Training Station ©2002Mubarik AliNo ratings yet
- Elefteria Psillakis, Vassilis Gekas (Auth.), Xavier Nicolay (Eds.) - Odors in The Food Industry-Springer US (2006)Document170 pagesElefteria Psillakis, Vassilis Gekas (Auth.), Xavier Nicolay (Eds.) - Odors in The Food Industry-Springer US (2006)Vipul JainNo ratings yet
- SM2016 - 32. 2150 Odor (1997)Document5 pagesSM2016 - 32. 2150 Odor (1997)David AmayaNo ratings yet
- FacultativeLagoonLiteratureReviewFINAL5292015 PDFDocument213 pagesFacultativeLagoonLiteratureReviewFINAL5292015 PDFHor SengNo ratings yet
- Odour Guidance UKDocument95 pagesOdour Guidance UKjsfernan3514100% (1)
- OdorizationDocument5 pagesOdorizationShreya Sahajpal KaushalNo ratings yet
- Principles of Desalination (Part A)From EverandPrinciples of Desalination (Part A)K SpeiglerNo ratings yet
- Titanium Spec - Chart PDFDocument1 pageTitanium Spec - Chart PDFClaudia MmsNo ratings yet
- Biotechniques for Air Pollution Abatement and Odour Control PoliciesFrom EverandBiotechniques for Air Pollution Abatement and Odour Control PoliciesNo ratings yet
- Water PinchDocument5 pagesWater PinchBruno CredidioNo ratings yet
- Condition of The Firetube Right After Removal From ServiceDocument1 pageCondition of The Firetube Right After Removal From ServiceClaudia MmsNo ratings yet
- Air Quality ModelingDocument34 pagesAir Quality ModelingabhinavNo ratings yet
- 35 New Euro Odor Standard PDFDocument16 pages35 New Euro Odor Standard PDFkrupali1579No ratings yet
- On The Unpredictability of OdorDocument8 pagesOn The Unpredictability of OdorFuckYouNo ratings yet
- EPRI Repair & Replacement Apps Center - Socket Weld Repair Issues 1013562Document20 pagesEPRI Repair & Replacement Apps Center - Socket Weld Repair Issues 1013562Claudia Mms100% (1)
- Aeration101 Scott Mulinix Presentation Rev1Document43 pagesAeration101 Scott Mulinix Presentation Rev1jvan migvelNo ratings yet
- RHDHV Industrial Water Treatment LR SpreadDocument11 pagesRHDHV Industrial Water Treatment LR SpreadgyfariNo ratings yet
- In Situ Repair Welding of Steam Turbine ShroudDocument7 pagesIn Situ Repair Welding of Steam Turbine ShroudClaudia Mms100% (1)
- AquaFit4Use - Water Quality Demands in Paper-Chemical-Food-Textile Industry PDFDocument125 pagesAquaFit4Use - Water Quality Demands in Paper-Chemical-Food-Textile Industry PDFVania Putri SenitaNo ratings yet
- Odour Filtration Range From BioactionDocument24 pagesOdour Filtration Range From BioactionLarry Botham100% (1)
- 0680 Air Pollution Engineering Manual Part1 1973Document110 pages0680 Air Pollution Engineering Manual Part1 1973gfrank9970No ratings yet
- Solidification Cracking in SS Welds Pe1119Document24 pagesSolidification Cracking in SS Welds Pe1119Claudia Mms100% (1)
- StainlessSteel Wire METCOLOY2Document6 pagesStainlessSteel Wire METCOLOY2Jarbas Moraes100% (1)
- Modular Treatment Approach for Drinking Water and WastewaterFrom EverandModular Treatment Approach for Drinking Water and WastewaterNo ratings yet
- Industrial Odor ControlDocument30 pagesIndustrial Odor ControlArindam BhowmickNo ratings yet
- Fluid Mixing II: A Symposium Organised by the Yorkshire Branch and the Fluid Mixing Processes Subject Group of the Institution of Chemical Engineers and Held at Bradford University, 3-5 April 1984From EverandFluid Mixing II: A Symposium Organised by the Yorkshire Branch and the Fluid Mixing Processes Subject Group of the Institution of Chemical Engineers and Held at Bradford University, 3-5 April 1984No ratings yet
- Odor PollutionDocument5 pagesOdor PollutionArafat IslamNo ratings yet
- Predicting OdourDocument9 pagesPredicting Odourshayna7No ratings yet
- Diagnostics and Control of Odours-General PresentationDocument36 pagesDiagnostics and Control of Odours-General PresentationwaxsswaxNo ratings yet
- Environmental ChemistryDocument20 pagesEnvironmental Chemistryajmermukhtar100% (2)
- Numerical Prediction of Fully-Suspended Slurry Flow in Horizontal PipesDocument10 pagesNumerical Prediction of Fully-Suspended Slurry Flow in Horizontal PipeskhanalhaniNo ratings yet
- Awwa C206Document74 pagesAwwa C206Jorge Navas VargasNo ratings yet
- Comparison of Jet Aeration Systems vs. Diffused Aeration SystemsDocument8 pagesComparison of Jet Aeration Systems vs. Diffused Aeration SystemsChristopher LloydNo ratings yet
- URLDocument21 pagesURLLyn Consing100% (3)
- George J.critsDocument55 pagesGeorge J.critsshaburo100% (1)
- CiVentiChem India Presentation - 2012Document60 pagesCiVentiChem India Presentation - 2012Venugopal Rao VeeramaneniNo ratings yet
- Principles of Desalination (Part B)From EverandPrinciples of Desalination (Part B)K.S. SpieglerNo ratings yet
- Dokumen - Tips Process Integration and Intensification Saving Energy Water and Resources 58adc6097048aDocument12 pagesDokumen - Tips Process Integration and Intensification Saving Energy Water and Resources 58adc6097048asamandondonNo ratings yet
- Centrifugation PDFDocument69 pagesCentrifugation PDFniezajanepatnaNo ratings yet
- Landfill Leachate Treatment Using Evaporation TechnologyDocument251 pagesLandfill Leachate Treatment Using Evaporation Technologypiter_martini3957100% (2)
- Ionac MembraneDocument2 pagesIonac Membranemarsur100% (1)
- Bio Degradation of PlasticsDocument24 pagesBio Degradation of PlasticsArchit Gupta100% (1)
- Carbon Dioxide Reduction Potential in The Global Cement Industry by 2050Document10 pagesCarbon Dioxide Reduction Potential in The Global Cement Industry by 2050Anonymous NxpnI6jCNo ratings yet
- Model Municipal Domestic Wastewater Management Plan PDFDocument20 pagesModel Municipal Domestic Wastewater Management Plan PDFTushy UciNo ratings yet
- Zero Liquid DischargeDocument3 pagesZero Liquid DischargeHendrix92McDowellNo ratings yet
- Factors Affecting Wet Scrubber PerformanceDocument4 pagesFactors Affecting Wet Scrubber PerformanceZee KayNo ratings yet
- Biosolids Treatment and ManagementDocument466 pagesBiosolids Treatment and ManagementDien Dang VanNo ratings yet
- An Introduction To MOP 21 - Automation of Wastewater Treatment FacilitiesDocument5 pagesAn Introduction To MOP 21 - Automation of Wastewater Treatment FacilitiesSugrit BuddhirakkulNo ratings yet
- Germantown Water Crisis ReviewDocument26 pagesGermantown Water Crisis ReviewLydian CoombsNo ratings yet
- Nitrification DenitrificationDocument4 pagesNitrification DenitrificationDon Javier HubbleNo ratings yet
- Innovations in Dewatering SludgesDocument2 pagesInnovations in Dewatering SludgesSebastian Gomez Betancourt100% (1)
- Biogas PDFDocument142 pagesBiogas PDFmracneaNo ratings yet
- Advantages and Limitations of Martensitic Steels For FusionDocument4 pagesAdvantages and Limitations of Martensitic Steels For FusionClaudia MmsNo ratings yet
- Expansion of The Sourcebook For Hydrogen Applications 03-10-2006Document1 pageExpansion of The Sourcebook For Hydrogen Applications 03-10-2006Claudia MmsNo ratings yet
- What Is Bellows Pressure Thrust PDFDocument2 pagesWhat Is Bellows Pressure Thrust PDFClaudia MmsNo ratings yet
- How To Become Certified by ASME 2010Document2 pagesHow To Become Certified by ASME 2010quiron2010No ratings yet
- An Introduction To Cathodic Protection PDFDocument5 pagesAn Introduction To Cathodic Protection PDFGERMAN1979No ratings yet
- DPVC 09 PetrobrasDocument47 pagesDPVC 09 PetrobrasClaudia MmsNo ratings yet
- Chemical Analysis Test Report: Element Sample T-1 Sample B-2 SAE/AISI Gr. A356.0Document2 pagesChemical Analysis Test Report: Element Sample T-1 Sample B-2 SAE/AISI Gr. A356.0Claudia MmsNo ratings yet
- FCAW Temper Bead Iamot - OrgDocument7 pagesFCAW Temper Bead Iamot - OrgClaudia MmsNo ratings yet
- WRC Bulletin No 506 Half-Bead Temper-BeadDocument2 pagesWRC Bulletin No 506 Half-Bead Temper-BeadClaudia MmsNo ratings yet
- Cronin 1981Document14 pagesCronin 1981glaucopzanellaNo ratings yet
- Brex Module1 - 9group 2BDocument32 pagesBrex Module1 - 9group 2BAnaliza Kitongan LantayanNo ratings yet
- Daftar Harga Power Supplay 2015Document26 pagesDaftar Harga Power Supplay 2015Syah J-RyanNo ratings yet
- Experiment No.04: Department of Chemistry Engineering Chemistry Laboratory Course CH1102Document2 pagesExperiment No.04: Department of Chemistry Engineering Chemistry Laboratory Course CH1102sam musicNo ratings yet
- Zhejiang Uniview Technologies Co., LTD.: Test Report No.: Page 1 of 31Document31 pagesZhejiang Uniview Technologies Co., LTD.: Test Report No.: Page 1 of 31rose_bellatrixNo ratings yet
- The Periodic TableDocument123 pagesThe Periodic TableFatema KhatunNo ratings yet
- Ch12 Redox Ws Keys 1 13Document28 pagesCh12 Redox Ws Keys 1 13Allen IBARRA VILLAMINNo ratings yet
- Model Answer For Second BookletDocument3 pagesModel Answer For Second BookletAnonymous ttyvxN4x83% (6)
- Chemical Reactions and Chemical EquationsDocument21 pagesChemical Reactions and Chemical EquationsdiofelNo ratings yet
- Csir Net Examination Chemical Sciences December 2012 PDFDocument57 pagesCsir Net Examination Chemical Sciences December 2012 PDFAbhay KumarNo ratings yet
- Periodic Table: Chemistry DPP 1 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Document3 pagesPeriodic Table: Chemistry DPP 1 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Yash Chopade100% (1)
- Preparation and Characterization of AlkeneDocument4 pagesPreparation and Characterization of AlkeneXyrell Claude Monta100% (2)
- 4.4 ElectrochemistryDocument37 pages4.4 ElectrochemistryDenisNo ratings yet
- Review On The Synthesis of LiNixMnyCo1-x-yO2 (NMC) Cathodes Forlithium Ion BatteriesDocument33 pagesReview On The Synthesis of LiNixMnyCo1-x-yO2 (NMC) Cathodes Forlithium Ion BatteriesMUHAMMAD ALIFNo ratings yet
- Method Descriptions Screen Assay ME SCR21Document2 pagesMethod Descriptions Screen Assay ME SCR21guanakhoNo ratings yet
- ImportsDocument9 pagesImportsParas SachanNo ratings yet
- Exercise No. 1 Mole To GramDocument5 pagesExercise No. 1 Mole To GramJoyae ChavezNo ratings yet
- Periodic Table FullDocument24 pagesPeriodic Table FullabydaieNo ratings yet
- Chemical Equations and Stoichiometry PDFDocument19 pagesChemical Equations and Stoichiometry PDFPanda MimiNo ratings yet
- Chemistry PresentationDocument43 pagesChemistry PresentationgabyyyyyyNo ratings yet
- Electrochemical Synthesis of Ammonia As ADocument4 pagesElectrochemical Synthesis of Ammonia As ADung Phan Thị ThùyNo ratings yet
- Der, Roa, Dantato. Returnsaham Ukuran PerusahaanDocument6 pagesDer, Roa, Dantato. Returnsaham Ukuran PerusahaanValdi IvanoNo ratings yet
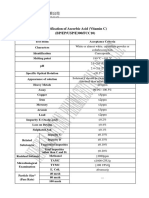
- Spec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangDocument2 pagesSpec (BP, EP, USP, E300, FCC) - Vitamin C Ex Ningxia QiyuangPaulo Roberto Baggio MoreiraNo ratings yet
- Anode CatalogueDocument161 pagesAnode CataloguePrabuNo ratings yet
- SAIC-W-2086 In-Process Welding Inspection PDFDocument5 pagesSAIC-W-2086 In-Process Welding Inspection PDFkarioke mohaNo ratings yet
- PREN Number Effect On AlloyDocument6 pagesPREN Number Effect On AlloyVed JoshiNo ratings yet
- CHM 477 Experiment 3 4 5 PDFDocument10 pagesCHM 477 Experiment 3 4 5 PDFAhmad ZakwanNo ratings yet
- Analys Lub Oil PDFDocument1 pageAnalys Lub Oil PDFdinar rosandyNo ratings yet