Professional Documents
Culture Documents
Rate of Reaction: Done By:-Ranim Khaled
Uploaded by
Maryam Raneem0 ratings0% found this document useful (0 votes)
3 views9 pagesm ,
Original Title
Rate of Reactionk
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentm ,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views9 pagesRate of Reaction: Done By:-Ranim Khaled
Uploaded by
Maryam Raneemm ,
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 9
Rate of reaction
Done by:-Ranim Khaled
Objectives: To be able to understand what is rate of
reaction
To be able to relate rate of reaction with
temperature , concentration and surface
area.
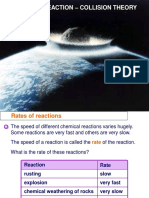
Rate of reaction: Therateof areactionis thespeedat which a
chemicalreactionhappens.
If areactionhas a lowrate, that means the molecules
combine at a slowerspeedthan areactionwith a high rate.
Somereactionstake hundreds, maybe even thousands, of
years while others can happen in less than one second.
Factors that affect the rate of the
reaction
1.Concentration
2.Temperature
3.Pressure
4.Surface area
5.catalysts
Temperature: When temperature increases,
energy of the particles will increase
, number of collisions will increase ,
number of successful collisions
increase finally the rate of reaction
increases.
Concentration
When concentation increases , number of particles increases ,
number of collision increases , number of successful collision
increases, rate of reaction increases.
When concentation decreases , number of particles
decreases , number of collision decreases, number of
successful collision decreases, rate of reaction decreases.
Surface Area: The rate of a chemical reaction can be raised by increasing
the surface area of a solid reactant. This is done by cutting
the substance into small pieces, or by grinding it into a
powder. If the surface area of a reactant is increased:
1. More particles are exposed to the other reactant
2. There are more collisions
3. The rate of reaction increases
You might also like
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersFrom EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersNo ratings yet
- 1a Rates of Reaction Slides PDFDocument26 pages1a Rates of Reaction Slides PDFArshad KhanNo ratings yet
- 2958 - Rate of ReactionDocument40 pages2958 - Rate of ReactioncorporolrexNo ratings yet
- Ate of Hemical Eactions: by StudentDocument17 pagesAte of Hemical Eactions: by StudentDyah Ayu pramoda wardaniNo ratings yet
- Collision TheoryDocument61 pagesCollision TheoryIda Yuni Sukadi50% (2)
- New Rate of Reaction Year 9Document19 pagesNew Rate of Reaction Year 9nwandu.elliot.ebenezerNo ratings yet
- Unit - The Periodic TableDocument20 pagesUnit - The Periodic TableMaurya AdeshraNo ratings yet
- CHEMICAL REACTIONSDocument100 pagesCHEMICAL REACTIONSmohidb642No ratings yet
- 10.3 Kinetic Factors Affecting-2Document54 pages10.3 Kinetic Factors Affecting-2Hafizszulfeyzul FeyzulNo ratings yet
- Factors Affecti-WPS OfficeDocument14 pagesFactors Affecti-WPS OfficeJessica TagaoNo ratings yet
- Rates of ReactionDocument31 pagesRates of Reactionspamzz063No ratings yet
- Rates CSEC-CAPEDocument26 pagesRates CSEC-CAPEKhadejah LawesNo ratings yet
- Chemical KineticsDocument25 pagesChemical KineticsAngelo PunzalanNo ratings yet
- Rates of ReactionsDocument55 pagesRates of ReactionsJOSHUA NYANGENANo ratings yet
- C8 Rates of ReactionDocument25 pagesC8 Rates of Reactionshayaanzaman0No ratings yet
- KINETICSDocument47 pagesKINETICSMarilia BonorinoNo ratings yet
- SPM Chemistry Form 4 Chapter 1Document37 pagesSPM Chemistry Form 4 Chapter 1kslpeter87No ratings yet
- KineticsDocument136 pagesKineticsStudent 365100% (1)
- Chemistry - Rates of ReactionDocument2 pagesChemistry - Rates of Reactionivanamoah20No ratings yet
- Collision Theory and Factors Affecting Reaction RatesDocument14 pagesCollision Theory and Factors Affecting Reaction RatesRasidah Abd SamatNo ratings yet
- Document 61Document2 pagesDocument 61Keandra JordanNo ratings yet
- Rates of Reactions FactorsDocument7 pagesRates of Reactions FactorsiamjebastinsamuelNo ratings yet
- Rates of ReactionDocument3 pagesRates of ReactionTofunmi OyeboluNo ratings yet
- Chemical KineticsDocument14 pagesChemical KineticsmochimochikoNo ratings yet
- Rate of ReactionDocument48 pagesRate of ReactionHAFSAH AHMADNo ratings yet
- Factors Affecti-WPS OfficeDocument14 pagesFactors Affecti-WPS OfficeJessica TagaoNo ratings yet
- Collision Theory - WJDocument16 pagesCollision Theory - WJFilzahtuln Farihah100% (1)
- Chemical Kinetics:: Reaction Rate and Collision RateDocument11 pagesChemical Kinetics:: Reaction Rate and Collision Rateズセプズ ジラーNo ratings yet
- Speed of Reaction SummaryDocument3 pagesSpeed of Reaction Summarychong5660% (5)
- Definition N FactorsDocument37 pagesDefinition N FactorsJedidah JongNo ratings yet
- Chemical ReactionDocument4 pagesChemical ReactionaleezaNo ratings yet
- 1-Chemical Kinetics First LectureDocument27 pages1-Chemical Kinetics First LecturealakaolamuhammadNo ratings yet
- Collision Theory - EditedDocument17 pagesCollision Theory - EditedrhaineNo ratings yet
- Reaction RateDocument9 pagesReaction RateKafitaNo ratings yet
- 1.5 KineticsDocument9 pages1.5 KineticsNina HSNo ratings yet
- Speed of Reactions PDFDocument8 pagesSpeed of Reactions PDFafoo1234No ratings yet
- Rate of ReactionDocument2 pagesRate of ReactionSiqi Luca LUONo ratings yet
- Physical Pharmacy Experiment 6Document5 pagesPhysical Pharmacy Experiment 6Krsna NaveraNo ratings yet
- Collision TheoryDocument15 pagesCollision TheoryEfraim KasinoNo ratings yet
- Rate of ReactionDocument19 pagesRate of Reactionkaila kilogramNo ratings yet
- Factors Affecting The Rate of ReactionDocument3 pagesFactors Affecting The Rate of Reactionapi-234891239100% (1)
- Rate of Reactions 18 April 2024(1)Document46 pagesRate of Reactions 18 April 2024(1)Amahle KudaNo ratings yet
- Collision Theory & CatalystDocument33 pagesCollision Theory & CatalystSHEENA MAE DALGUNTASNo ratings yet
- Kinetics and Equilibria: Factors Affecting Reaction RatesDocument57 pagesKinetics and Equilibria: Factors Affecting Reaction RatesJana Abo elfotohNo ratings yet
- Chemical Kinetics & Equilibrium: Froilan Aron S. Faraon, R.PHDocument34 pagesChemical Kinetics & Equilibrium: Froilan Aron S. Faraon, R.PHKenneth TrogonNo ratings yet
- Lajur FixDocument18 pagesLajur FixMoniqsa Purbo SyahraniNo ratings yet
- Short Note Chemistry Form 5-Chapter 1 Rate of ReactionDocument4 pagesShort Note Chemistry Form 5-Chapter 1 Rate of Reactionsalamah_sabri100% (1)
- Rate of Reaction and Various Factors That Influence ItDocument28 pagesRate of Reaction and Various Factors That Influence Itjulie cadungonNo ratings yet
- Powerpoint - Collision Theory and Factors Affecting Rate of ReactionDocument25 pagesPowerpoint - Collision Theory and Factors Affecting Rate of ReactionAref DahabrahNo ratings yet
- The Rate of Chemical Reaction - NotesDocument6 pagesThe Rate of Chemical Reaction - Notesshawaiz.m.malik2010No ratings yet
- Introduction To Chemical KineticsDocument19 pagesIntroduction To Chemical KineticsGodwin EdekheNo ratings yet
- Introduction To Chemical KineticsDocument19 pagesIntroduction To Chemical KineticsGodwin EdekheNo ratings yet
- Gen Chem Group 4 Factors Affecting The Reaction Rates Autosaved 1Document12 pagesGen Chem Group 4 Factors Affecting The Reaction Rates Autosaved 1Roselie DuldulaoNo ratings yet
- Rate of ReactionDocument23 pagesRate of ReactionVirly vcNo ratings yet
- Chemical Change 1Document36 pagesChemical Change 1CheloGraceTiozonAmparadoNo ratings yet
- SHS Core - Earth Science CGDocument13 pagesSHS Core - Earth Science CGNuevalyn Quijano FernandoNo ratings yet
- Rate of ReactionDocument12 pagesRate of ReactionRobin CabralNo ratings yet
- Chapter 8.1 Kinetics and EquilibriumDocument20 pagesChapter 8.1 Kinetics and EquilibriumdawsontangxyNo ratings yet
- Factors Affecting The Rate of ReactionDocument19 pagesFactors Affecting The Rate of ReactionRasidah Abd Samat100% (1)