Professional Documents
Culture Documents
Guofu Chen Oxygen Separation Liquefaction ASU Perry Exergy Thermodynamic Analysis
Guofu Chen Oxygen Separation Liquefaction ASU Perry Exergy Thermodynamic Analysis
Uploaded by
chenguofuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Guofu Chen Oxygen Separation Liquefaction ASU Perry Exergy Thermodynamic Analysis
Guofu Chen Oxygen Separation Liquefaction ASU Perry Exergy Thermodynamic Analysis
Uploaded by
chenguofuCopyright:
Available Formats
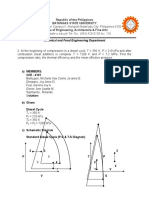
TABLE 4-2 States and Values of Properties for the Process of Fig.
4-12*
Point
P (bar)
T (K)
Composition
State
H (J/mol)
1
55.22
300
Air
Superheated
12,046
2
1.01
295
Pure O2
Superheated
13,460
3
1.01
295
91.48% N2
Superheated
12,074
4
55.22
147.2
Air
Superheated
5,850
5
1.01
79.4
91.48% N2
Saturated vapor
5,773
6
1.01
90
pure O2
Saturated vapor
7,485
7
1.01
300
Air
Superheated
12,407
*Properties on the basis of Miller and Sullivan, U.S. Bur. Mines Tech. Pap. 424 (1928).
T
Comp stage
Comp isentropic eff
Compressor
Ideal work
Compressor loss
Exchanger loss
Column loss
Note:
(K)
J
J
J
J
J
300
3
80%
15171
Compressor power
590
5221
2073
7288
15171
3.9%
34.4%
13.7%
48.0%
100.0%
S (J/mol/K)
82.98
118.48
114.34
52.08
75.82
83.69
117.35
Flow (mol) Exergy (J/mol) Exergy (J)
1
-12848
-12848
0.1364
-22084
-3012
0.8636
-22228
-19196
1
-9774
-9774
0.8636
-16973
-14658
0.1364
-17622
-2404
1
-22798
-22798
Point
1
2
3
4
5
6
7
E means exergy, number means Point. Not mean excel cell reference
= E2 + E3 - E7
= Compressor power - (E1 - E7)
= E1 + E5 + E6 - E2 - E3 - E4
= E4 - E5 - E6
You can find this calculation in Perry's Chemical Engineers' Handbook (7th edition, Chapter 4 Thermodynamics, Page 4-36)
In the Perry's calculation, Exergy lost = T * S
While in my calculation, Exergy lost = Exergy in - Exergy out. It's easiler to understand and the calculations is also faster.
Copyright Protected. More informaiton can be found at
www.guofuchen.com
Chen_Calculation / 272926105.xlsx
You might also like
- Thermodynamic Tables SIDocument43 pagesThermodynamic Tables SIPavirlene Escaño NorteNo ratings yet
- Rac AssignmentDocument21 pagesRac AssignmentJagdeep SinghNo ratings yet
- Miftahul Ulfa (1407113349) Tugas Termidinamika Ke-3Document5 pagesMiftahul Ulfa (1407113349) Tugas Termidinamika Ke-3WinterblueeNo ratings yet
- Modeling A Crude Vacuum System With Preheat TrainDocument6 pagesModeling A Crude Vacuum System With Preheat TrainELTIPAZONo ratings yet
- Compressor Technical DataDocument3 pagesCompressor Technical DataaushinNo ratings yet
- Cooling TowerDocument14 pagesCooling TowerAbdulla DoskiNo ratings yet
- Thermodynamic Tables English UnitsDocument42 pagesThermodynamic Tables English UnitsShankar Dakshinamurthi100% (1)
- CP (T) DT: AIR FuelDocument3 pagesCP (T) DT: AIR FuelPriambudi PujihatmaNo ratings yet
- AbstractDocument7 pagesAbstractSyazwani AbdullahNo ratings yet
- THD291Z 2009 10 e 1Document15 pagesTHD291Z 2009 10 e 1kasturiep15No ratings yet
- ThermodynamicDocument50 pagesThermodynamicSkander El AmriNo ratings yet
- Physical Properties of Some Common Refrigerants Are IndicatedDocument8 pagesPhysical Properties of Some Common Refrigerants Are IndicatedRajeshSekarNo ratings yet
- Remote Desktop Redirected PrinterDocument1 pageRemote Desktop Redirected PrinterBen VaughtNo ratings yet
- Thermodynamics Table - Cengel PDFDocument82 pagesThermodynamics Table - Cengel PDFFachransjah Aliunir0% (1)
- SMRPRICO - Plus - HYSYS (Aspentech)Document8 pagesSMRPRICO - Plus - HYSYS (Aspentech)Ian MannNo ratings yet
- Tables and Index Thermodynamics Cengel 7E-2Document118 pagesTables and Index Thermodynamics Cengel 7E-2tomtom9649No ratings yet
- Production of 80,000 Mtpa of Benzene, Toluene and Xylene (BTX) From PygasDocument19 pagesProduction of 80,000 Mtpa of Benzene, Toluene and Xylene (BTX) From PygasCalvin Lin Jia RongNo ratings yet
- Thermophysical Properties: T H o M A S F. Irvine JRDocument74 pagesThermophysical Properties: T H o M A S F. Irvine JRAbu Izzan Al BunyNo ratings yet
- CH 4 PDFDocument8 pagesCH 4 PDFAbdulbari UshNo ratings yet
- 1 HáziottoDocument10 pages1 HáziottoHuan TranNo ratings yet
- Power Plant Technology, El Wakil, Problem 8.14Document33 pagesPower Plant Technology, El Wakil, Problem 8.14Reynold Curampez100% (2)
- MCG 2131 Exam 08Document6 pagesMCG 2131 Exam 08子豪王No ratings yet
- HYSYS-Print Tugas 4 PDFDocument1 pageHYSYS-Print Tugas 4 PDFAde HadyNo ratings yet
- LaRoche NH3 Tech Data ManualDocument26 pagesLaRoche NH3 Tech Data ManualWojciech ŻaczekNo ratings yet
- Biomass PowerplantDocument13 pagesBiomass PowerplantZohre AlinejadNo ratings yet
- Design of Pump After DryerDocument3 pagesDesign of Pump After DryerNovia Mia YuhermitaNo ratings yet
- Exergía Acido NitricoDocument35 pagesExergía Acido NitricoAnita BuelvasNo ratings yet
- Modelling & Simulation Lab Assignment: Submitted By: H. Saad Naseer 2011-CH-53Document8 pagesModelling & Simulation Lab Assignment: Submitted By: H. Saad Naseer 2011-CH-53Abdul Mannan KhanNo ratings yet
- Technical Data Sheet: Features and Uses of R-407CDocument4 pagesTechnical Data Sheet: Features and Uses of R-407Ccala ingenieriaNo ratings yet
- Experiment No 8 DP 2Document28 pagesExperiment No 8 DP 2Drw ArcyNo ratings yet
- Tabel-Thermo Gas Hasil Pmbakaran Cengel (SI-18 HLM)Document18 pagesTabel-Thermo Gas Hasil Pmbakaran Cengel (SI-18 HLM)rasid redNo ratings yet
- A + B (T) + C (T 2) + D (T 3) + E (T 4) + F (T 5) : 1. Mixing 2. Feeding Tank FermentorDocument8 pagesA + B (T) + C (T 2) + D (T 3) + E (T 4) + F (T 5) : 1. Mixing 2. Feeding Tank FermentormaritsyaditaaNo ratings yet
- Entalpija Produkata Sagorjevanja Produkti Sagorjevanja Vazduh K.P.SDocument8 pagesEntalpija Produkata Sagorjevanja Produkti Sagorjevanja Vazduh K.P.SBoris StjepicNo ratings yet
- Super Critical PresentationDocument46 pagesSuper Critical PresentationSam100% (1)
- 4200:225 Equilibrium Thermodynamics: Unit I. Earth, Air, Fire, and WaterDocument11 pages4200:225 Equilibrium Thermodynamics: Unit I. Earth, Air, Fire, and WaterRiky IkhwanNo ratings yet
- Escuela Politécnica Nacional: Facultad de Ingeniería MecánicaDocument14 pagesEscuela Politécnica Nacional: Facultad de Ingeniería MecánicaAly HerreraNo ratings yet
- Written Solution ReportDocument5 pagesWritten Solution ReportmichsantosNo ratings yet
- Nitric AcidDocument14 pagesNitric Acidmalini2201No ratings yet
- Appendix2 EnglishDocument42 pagesAppendix2 EnglishSantanu BiswasNo ratings yet
- Simulation of Vapour Compression CycleDocument31 pagesSimulation of Vapour Compression CycleAlessandro LamaNo ratings yet
- Sim 3Document2 pagesSim 3Maywathan LinNo ratings yet
- Engineering Declaration Unit # 2 JindalDocument16 pagesEngineering Declaration Unit # 2 JindalVIBHAV100% (1)
- Heater 1: Kapasitas Panas Rumus Molekul Nama A B C DDocument17 pagesHeater 1: Kapasitas Panas Rumus Molekul Nama A B C DAndri ZalNo ratings yet
- Of College of Chemical: Classical ClassDocument4 pagesOf College of Chemical: Classical Classنزار الدهاميNo ratings yet
- Lab Report On Cooling Tower: Performed byDocument9 pagesLab Report On Cooling Tower: Performed byramesh pokhrelNo ratings yet
- Table A-2: Pressure Conv Ersions: 1 Bar 0.1 Mpa 10 KpaDocument10 pagesTable A-2: Pressure Conv Ersions: 1 Bar 0.1 Mpa 10 KpaeyeerrNo ratings yet
- Water Tables MoranDocument10 pagesWater Tables MorantherealxbladeNo ratings yet
- Chlorine NISTDocument5 pagesChlorine NISTzhyhhNo ratings yet
- Negara Maju UpdDocument10 pagesNegara Maju UpdKapibaraNo ratings yet
- Appendix D: Ideal Gas & Incompressible Substances: MW R C C KDocument1 pageAppendix D: Ideal Gas & Incompressible Substances: MW R C C KjanNo ratings yet
- Analysis Using Various Approaches For Residual Life Estimation of Power TransformersDocument19 pagesAnalysis Using Various Approaches For Residual Life Estimation of Power TransformersJicheng PiaoNo ratings yet
- He DefinitionsDocument2 pagesHe DefinitionsAnibal GonzalezNo ratings yet
- Bab Iv Neraca Energi: 4.1 Fermenter (R-101)Document3 pagesBab Iv Neraca Energi: 4.1 Fermenter (R-101)Dewi AnggrainiNo ratings yet
- Specific Heats For Steam, Water, Air & Flue GasesDocument5 pagesSpecific Heats For Steam, Water, Air & Flue GasesRagu NathanNo ratings yet
- Fix Banget NM Sama NP Yosua Olin Final - 16 DesemberDocument153 pagesFix Banget NM Sama NP Yosua Olin Final - 16 DesemberWilly DinataNo ratings yet
- Fundamentals of Engineering Thermodynamics 2nbsped 812032790x 9788120327900 CompressDocument720 pagesFundamentals of Engineering Thermodynamics 2nbsped 812032790x 9788120327900 Compresssweetyjun161998No ratings yet
- Exp - 6 - Carbothermal Reductive MeltingDocument7 pagesExp - 6 - Carbothermal Reductive MeltingIbrahim MücahitNo ratings yet
- Sustainable Energy Conversion for Electricity and Coproducts: Principles, Technologies, and EquipmentFrom EverandSustainable Energy Conversion for Electricity and Coproducts: Principles, Technologies, and EquipmentNo ratings yet
- Synthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsFrom EverandSynthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsTilman J. SchildhauerNo ratings yet
- CCS Abstract SubmissionDocument1 pageCCS Abstract SubmissionchenguofuNo ratings yet
- How To Model Dry Ice (CO2) CoolingDocument1 pageHow To Model Dry Ice (CO2) CoolingchenguofuNo ratings yet
- Chem Therm 650Document2 pagesChem Therm 650chenguofuNo ratings yet
- 08 PRV SizingDocument117 pages08 PRV SizingchenguofuNo ratings yet
- CheGuide Beggs & Brill MethodDocument6 pagesCheGuide Beggs & Brill MethodchenguofuNo ratings yet
- Two Phase Flow Regime Correlations ProMaxDocument6 pagesTwo Phase Flow Regime Correlations ProMaxchenguofuNo ratings yet
- ProMax PipeDocument23 pagesProMax PipechenguofuNo ratings yet
- AMACS DeMister Randing PackingDocument126 pagesAMACS DeMister Randing PackingchenguofuNo ratings yet
- United States Patent (19) : (73) AssigneeDocument7 pagesUnited States Patent (19) : (73) AssigneechenguofuNo ratings yet
- Unveil The Mystery of Air Products APCI C3MR LNG Liquefaction Process in HYSYSDocument2 pagesUnveil The Mystery of Air Products APCI C3MR LNG Liquefaction Process in HYSYSchenguofuNo ratings yet
- Power Saving of Liquid Expander Rev1 ToBe UpdatedDocument3 pagesPower Saving of Liquid Expander Rev1 ToBe UpdatedchenguofuNo ratings yet
- Promax Custom Report Template Save Versi 1 Scope Logic Comment Worksheet Jtv000 Err:509 Jtv001Document4 pagesPromax Custom Report Template Save Versi 1 Scope Logic Comment Worksheet Jtv000 Err:509 Jtv001chenguofuNo ratings yet