Professional Documents
Culture Documents
Chapter 3: Oxidation and Reduction Structure Questions 1
Chapter 3: Oxidation and Reduction Structure Questions 1
Uploaded by
ChrisLeeCopyright:
Available Formats
You might also like
- 2012 OLevel Pure Chemistry Paper 2 Questions and AnswersDocument13 pages2012 OLevel Pure Chemistry Paper 2 Questions and AnswersMethodology OfStudies100% (6)
- Acids Bases and Salts Worksheet 1Document2 pagesAcids Bases and Salts Worksheet 1api-251783882100% (3)
- Acids and Bases - HL - 002: (153 Marks)Document36 pagesAcids and Bases - HL - 002: (153 Marks)VedantNo ratings yet
- IGCSE Chemistry - Topic 4 Test: - Multiple Choice (10 Marks)Document9 pagesIGCSE Chemistry - Topic 4 Test: - Multiple Choice (10 Marks)Yunsik HanNo ratings yet
- Trial STPM Term2 2015Document8 pagesTrial STPM Term2 2015Earliany Mohd ShahriNo ratings yet
- Bengkel Ambang SPM 2009 Kertas 2Document31 pagesBengkel Ambang SPM 2009 Kertas 2azharsarahNo ratings yet
- Oxidation and ReductionDocument8 pagesOxidation and ReductionHAKIMIN_KHAIRUL3674No ratings yet
- Victoria Junior College JC 2 Preliminary Examinations Higher 2Document11 pagesVictoria Junior College JC 2 Preliminary Examinations Higher 2Jing Yi KuahNo ratings yet
- Ejercitacion N1Document3 pagesEjercitacion N1Seba PalopoliNo ratings yet
- CBSE Class 12 Chemistry Sample Paper-04 (For 2014)Document6 pagesCBSE Class 12 Chemistry Sample Paper-04 (For 2014)cbsestudymaterialsNo ratings yet
- Bab 3 Kertas 1Document15 pagesBab 3 Kertas 1Siva GuruNo ratings yet
- IBO Worksheet ChemistryDocument26 pagesIBO Worksheet ChemistryAarav PatelNo ratings yet
- IMP Question Bank Class XIIDocument8 pagesIMP Question Bank Class XIIeshani0706No ratings yet
- QuestionsDocument16 pagesQuestionsPhan Do Dang KhoaNo ratings yet
- ISC Board Question Paper Class XII - 2009Document5 pagesISC Board Question Paper Class XII - 2009Biswajit GhoshNo ratings yet
- Transition Metals H2 QuestionsDocument7 pagesTransition Metals H2 QuestionskitoniumNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 12Document8 pagesRTS Chemistry SPM Question Bank Chapter 12dobbybibiNo ratings yet
- 2011 Chemistry Question PapersDocument4 pages2011 Chemistry Question Papersalex scottNo ratings yet
- The Jammu & Kashmir State Board of School Education0Document4 pagesThe Jammu & Kashmir State Board of School Education0Shah JunaidNo ratings yet
- Topic 3/13 Practice IB Chem TestDocument12 pagesTopic 3/13 Practice IB Chem TestKeyerria HowardNo ratings yet
- ChemistryDocument2 pagesChemistryabeltesfaye98No ratings yet
- 11 Chemistry CBSE Redox ReactionsDocument2 pages11 Chemistry CBSE Redox ReactionsNitesh GuptaNo ratings yet
- Shivam Sir Immortal Chemistry Academy Chemistry 12 Imp. Q.Document5 pagesShivam Sir Immortal Chemistry Academy Chemistry 12 Imp. Q.Mansi OjhaNo ratings yet
- Chapter 13 Transition Elements ExerciseDocument6 pagesChapter 13 Transition Elements Exerciseisqma100% (1)
- 2011 H2 Chem ACJC Prelim Paper 2Document16 pages2011 H2 Chem ACJC Prelim Paper 2onnoez0% (1)
- STPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)Document13 pagesSTPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)kokpin100100% (1)
- Aqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04Document14 pagesAqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04thc8477No ratings yet
- Chemistry PQMSDocument10 pagesChemistry PQMSprincesingh052005No ratings yet
- Nov 2008Document13 pagesNov 2008dharshanaabNo ratings yet
- Redox WorksheetDocument3 pagesRedox WorksheetMin Chong100% (2)
- Chemistry Chapter 1Document9 pagesChemistry Chapter 1Princy Merin JoseNo ratings yet
- Solution 1:: Chemical Reactions and EquationsDocument9 pagesSolution 1:: Chemical Reactions and EquationsOjasNo ratings yet
- ChemistryDocument32 pagesChemistry190519123No ratings yet
- 2010 NYJC 9647 H2 Chem Paper 3 AnswersDocument25 pages2010 NYJC 9647 H2 Chem Paper 3 AnswersYeeloong YlNo ratings yet
- Time: 3.00 Hours) : This Question Paper Contains 8 Printed PagesDocument8 pagesTime: 3.00 Hours) : This Question Paper Contains 8 Printed PagesrafikdmeNo ratings yet
- Item Kbat Chemistry Form Four Structure of The AtomDocument35 pagesItem Kbat Chemistry Form Four Structure of The AtomSiva GuruNo ratings yet
- Surface Chemistry ExercisesDocument19 pagesSurface Chemistry ExercisesShivang K RaghuvanshiNo ratings yet
- Unit 5 Practical 7 - Reactions of Transition Metal Complex IonsDocument1 pageUnit 5 Practical 7 - Reactions of Transition Metal Complex IonsstefyfoyerNo ratings yet
- Kendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15Document5 pagesKendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15NareshNo ratings yet
- 2021 - Boi Duong e-KHTN - Chem - Huy - HS - 3Document14 pages2021 - Boi Duong e-KHTN - Chem - Huy - HS - 3Thành Danh NguyễnNo ratings yet
- 2003 Blakehurst High School Chemistry Half Yearly ExamDocument9 pages2003 Blakehurst High School Chemistry Half Yearly ExamJay LiNo ratings yet
- AP Chemistry 1999 With AnswersDocument22 pagesAP Chemistry 1999 With AnswersjhbmleeNo ratings yet
- Chemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Document55 pagesChemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Apple KWNo ratings yet
- 5.2 (152 Marks) : 1. (1 Mark)Document42 pages5.2 (152 Marks) : 1. (1 Mark)Semwezi EnockNo ratings yet
- Class 11 Chemistry Sample PaperDocument6 pagesClass 11 Chemistry Sample PaperDamodar KasukurthiNo ratings yet
- s4 Chemistry Paper 2 Set 4 Marking Guide 1Document13 pagess4 Chemistry Paper 2 Set 4 Marking Guide 1Namuli MercyNo ratings yet
- Read The Given Passage and Answer The Questions 1 To 5 That FollowDocument4 pagesRead The Given Passage and Answer The Questions 1 To 5 That Followshafi hamzaNo ratings yet
- Exam t2 2011.12 Chemistry f6 p1Document10 pagesExam t2 2011.12 Chemistry f6 p1asjawolverineNo ratings yet
- Chemistry 2019Document7 pagesChemistry 2019HARSH MAHTONo ratings yet
- Chemistry (233) Mind JogDocument23 pagesChemistry (233) Mind JogNishad EsdorNo ratings yet
- Chemistry: InstructionsDocument3 pagesChemistry: InstructionsVenu GopalNo ratings yet
- Chemistry MSDocument6 pagesChemistry MSUtkarsh SharmaNo ratings yet
- I Just Want TP Fucking Download ShjitDocument10 pagesI Just Want TP Fucking Download ShjitMisfortuneEdward L.No ratings yet
- Full Chemistry Board Exam Pattern TestDocument8 pagesFull Chemistry Board Exam Pattern TestRanjanNo ratings yet
- Xi Chemistry Set 4Document6 pagesXi Chemistry Set 4aashirwad2076No ratings yet
- Ea7145df 2Document5 pagesEa7145df 2KevinNo ratings yet
- 2 Metal Complex EqDocument14 pages2 Metal Complex EqpogiatmagagandaNo ratings yet
- Sarawak Chemistry SPM Trial Exam 2011Document69 pagesSarawak Chemistry SPM Trial Exam 2011Philip TiongNo ratings yet
- Ion Exchange in Environmental Processes: Fundamentals, Applications and Sustainable TechnologyFrom EverandIon Exchange in Environmental Processes: Fundamentals, Applications and Sustainable TechnologyNo ratings yet
- 11Document2 pages11ChrisLeeNo ratings yet
- Chapter 4: Thermochemistry Objective Questions: Pengetahuan TinggiDocument1 pageChapter 4: Thermochemistry Objective Questions: Pengetahuan TinggiChrisLeeNo ratings yet
- Experimen T Set-Up of Apparatus ObservationDocument1 pageExperimen T Set-Up of Apparatus ObservationChrisLeeNo ratings yet
- By Referring To The Graph, Explain How Auxin Affects The Growth of Shoot and Roots. Shoot: Root: (2 Marks)Document1 pageBy Referring To The Graph, Explain How Auxin Affects The Growth of Shoot and Roots. Shoot: Root: (2 Marks)ChrisLeeNo ratings yet
- Growth Response of Organ To Applied Auxin Root - Shoot ShootDocument1 pageGrowth Response of Organ To Applied Auxin Root - Shoot ShootChrisLeeNo ratings yet
- Diagram 2.1 Shows A Human BrainDocument1 pageDiagram 2.1 Shows A Human BrainChrisLeeNo ratings yet
Chapter 3: Oxidation and Reduction Structure Questions 1
Chapter 3: Oxidation and Reduction Structure Questions 1
Uploaded by
ChrisLeeOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 3: Oxidation and Reduction Structure Questions 1
Chapter 3: Oxidation and Reduction Structure Questions 1
Uploaded by
ChrisLeeCopyright:
Available Formats
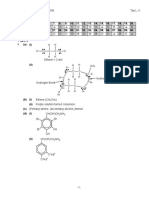
CHAPTER 3: OXIDATION AND REDUCTION
STRUCTURE QUESTIONS
1
Diagram 1 shows the set- up of apparatus to investigate the reaction between
potassium iodide solution and chlorine water through the transfer of electrons at a
distance..
G
Electrode P
Electrode Q
Potassium iodide solution
Chlorine water
Dilute sulphuric acid
DIAGRAM 1
a) What is the function of dilute sulphuric acid? [1 mark ]
b) On the diagram 1, draw the direction of the flow of electrons. [1 mark ]
c) (i) What is the colour change in the solution around electrode P? [1 mark ]
(ii) Describe a chemical test to determine the product formed in the solution
at
electrode P. [2 marks ]
d) What is the substance that is being oxidised in the experiment? Explain why.
[2 marks ]
e) Write a half equation for the reaction that occurs at electrode Q. [1 mark]
f) Suggest another reagent that can replace chlorine water. [1 mark]
g) What is the change in oxidation number of chlorine in the reaction? [1 mark]
You might also like
- 2012 OLevel Pure Chemistry Paper 2 Questions and AnswersDocument13 pages2012 OLevel Pure Chemistry Paper 2 Questions and AnswersMethodology OfStudies100% (6)
- Acids Bases and Salts Worksheet 1Document2 pagesAcids Bases and Salts Worksheet 1api-251783882100% (3)
- Acids and Bases - HL - 002: (153 Marks)Document36 pagesAcids and Bases - HL - 002: (153 Marks)VedantNo ratings yet
- IGCSE Chemistry - Topic 4 Test: - Multiple Choice (10 Marks)Document9 pagesIGCSE Chemistry - Topic 4 Test: - Multiple Choice (10 Marks)Yunsik HanNo ratings yet
- Trial STPM Term2 2015Document8 pagesTrial STPM Term2 2015Earliany Mohd ShahriNo ratings yet
- Bengkel Ambang SPM 2009 Kertas 2Document31 pagesBengkel Ambang SPM 2009 Kertas 2azharsarahNo ratings yet
- Oxidation and ReductionDocument8 pagesOxidation and ReductionHAKIMIN_KHAIRUL3674No ratings yet
- Victoria Junior College JC 2 Preliminary Examinations Higher 2Document11 pagesVictoria Junior College JC 2 Preliminary Examinations Higher 2Jing Yi KuahNo ratings yet
- Ejercitacion N1Document3 pagesEjercitacion N1Seba PalopoliNo ratings yet
- CBSE Class 12 Chemistry Sample Paper-04 (For 2014)Document6 pagesCBSE Class 12 Chemistry Sample Paper-04 (For 2014)cbsestudymaterialsNo ratings yet
- Bab 3 Kertas 1Document15 pagesBab 3 Kertas 1Siva GuruNo ratings yet
- IBO Worksheet ChemistryDocument26 pagesIBO Worksheet ChemistryAarav PatelNo ratings yet
- IMP Question Bank Class XIIDocument8 pagesIMP Question Bank Class XIIeshani0706No ratings yet
- QuestionsDocument16 pagesQuestionsPhan Do Dang KhoaNo ratings yet
- ISC Board Question Paper Class XII - 2009Document5 pagesISC Board Question Paper Class XII - 2009Biswajit GhoshNo ratings yet
- Transition Metals H2 QuestionsDocument7 pagesTransition Metals H2 QuestionskitoniumNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 12Document8 pagesRTS Chemistry SPM Question Bank Chapter 12dobbybibiNo ratings yet
- 2011 Chemistry Question PapersDocument4 pages2011 Chemistry Question Papersalex scottNo ratings yet
- The Jammu & Kashmir State Board of School Education0Document4 pagesThe Jammu & Kashmir State Board of School Education0Shah JunaidNo ratings yet
- Topic 3/13 Practice IB Chem TestDocument12 pagesTopic 3/13 Practice IB Chem TestKeyerria HowardNo ratings yet
- ChemistryDocument2 pagesChemistryabeltesfaye98No ratings yet
- 11 Chemistry CBSE Redox ReactionsDocument2 pages11 Chemistry CBSE Redox ReactionsNitesh GuptaNo ratings yet
- Shivam Sir Immortal Chemistry Academy Chemistry 12 Imp. Q.Document5 pagesShivam Sir Immortal Chemistry Academy Chemistry 12 Imp. Q.Mansi OjhaNo ratings yet
- Chapter 13 Transition Elements ExerciseDocument6 pagesChapter 13 Transition Elements Exerciseisqma100% (1)
- 2011 H2 Chem ACJC Prelim Paper 2Document16 pages2011 H2 Chem ACJC Prelim Paper 2onnoez0% (1)
- STPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)Document13 pagesSTPM Johor Chemistry Paper 2 2011 Trial From (Edu - Joshuatly)kokpin100100% (1)
- Aqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04Document14 pagesAqueous Hydrogen Peroxide: Ó UCLES 2004 5070/01/M/J/04thc8477No ratings yet
- Chemistry PQMSDocument10 pagesChemistry PQMSprincesingh052005No ratings yet
- Nov 2008Document13 pagesNov 2008dharshanaabNo ratings yet
- Redox WorksheetDocument3 pagesRedox WorksheetMin Chong100% (2)
- Chemistry Chapter 1Document9 pagesChemistry Chapter 1Princy Merin JoseNo ratings yet
- Solution 1:: Chemical Reactions and EquationsDocument9 pagesSolution 1:: Chemical Reactions and EquationsOjasNo ratings yet
- ChemistryDocument32 pagesChemistry190519123No ratings yet
- 2010 NYJC 9647 H2 Chem Paper 3 AnswersDocument25 pages2010 NYJC 9647 H2 Chem Paper 3 AnswersYeeloong YlNo ratings yet
- Time: 3.00 Hours) : This Question Paper Contains 8 Printed PagesDocument8 pagesTime: 3.00 Hours) : This Question Paper Contains 8 Printed PagesrafikdmeNo ratings yet
- Item Kbat Chemistry Form Four Structure of The AtomDocument35 pagesItem Kbat Chemistry Form Four Structure of The AtomSiva GuruNo ratings yet
- Surface Chemistry ExercisesDocument19 pagesSurface Chemistry ExercisesShivang K RaghuvanshiNo ratings yet
- Unit 5 Practical 7 - Reactions of Transition Metal Complex IonsDocument1 pageUnit 5 Practical 7 - Reactions of Transition Metal Complex IonsstefyfoyerNo ratings yet
- Kendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15Document5 pagesKendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15NareshNo ratings yet
- 2021 - Boi Duong e-KHTN - Chem - Huy - HS - 3Document14 pages2021 - Boi Duong e-KHTN - Chem - Huy - HS - 3Thành Danh NguyễnNo ratings yet
- 2003 Blakehurst High School Chemistry Half Yearly ExamDocument9 pages2003 Blakehurst High School Chemistry Half Yearly ExamJay LiNo ratings yet
- AP Chemistry 1999 With AnswersDocument22 pagesAP Chemistry 1999 With AnswersjhbmleeNo ratings yet
- Chemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Document55 pagesChemistry Pahang JUJ 2008 (Edu - Joshuatly.com)Apple KWNo ratings yet
- 5.2 (152 Marks) : 1. (1 Mark)Document42 pages5.2 (152 Marks) : 1. (1 Mark)Semwezi EnockNo ratings yet
- Class 11 Chemistry Sample PaperDocument6 pagesClass 11 Chemistry Sample PaperDamodar KasukurthiNo ratings yet
- s4 Chemistry Paper 2 Set 4 Marking Guide 1Document13 pagess4 Chemistry Paper 2 Set 4 Marking Guide 1Namuli MercyNo ratings yet
- Read The Given Passage and Answer The Questions 1 To 5 That FollowDocument4 pagesRead The Given Passage and Answer The Questions 1 To 5 That Followshafi hamzaNo ratings yet
- Exam t2 2011.12 Chemistry f6 p1Document10 pagesExam t2 2011.12 Chemistry f6 p1asjawolverineNo ratings yet
- Chemistry 2019Document7 pagesChemistry 2019HARSH MAHTONo ratings yet
- Chemistry (233) Mind JogDocument23 pagesChemistry (233) Mind JogNishad EsdorNo ratings yet
- Chemistry: InstructionsDocument3 pagesChemistry: InstructionsVenu GopalNo ratings yet
- Chemistry MSDocument6 pagesChemistry MSUtkarsh SharmaNo ratings yet
- I Just Want TP Fucking Download ShjitDocument10 pagesI Just Want TP Fucking Download ShjitMisfortuneEdward L.No ratings yet
- Full Chemistry Board Exam Pattern TestDocument8 pagesFull Chemistry Board Exam Pattern TestRanjanNo ratings yet
- Xi Chemistry Set 4Document6 pagesXi Chemistry Set 4aashirwad2076No ratings yet
- Ea7145df 2Document5 pagesEa7145df 2KevinNo ratings yet
- 2 Metal Complex EqDocument14 pages2 Metal Complex EqpogiatmagagandaNo ratings yet
- Sarawak Chemistry SPM Trial Exam 2011Document69 pagesSarawak Chemistry SPM Trial Exam 2011Philip TiongNo ratings yet
- Ion Exchange in Environmental Processes: Fundamentals, Applications and Sustainable TechnologyFrom EverandIon Exchange in Environmental Processes: Fundamentals, Applications and Sustainable TechnologyNo ratings yet
- 11Document2 pages11ChrisLeeNo ratings yet
- Chapter 4: Thermochemistry Objective Questions: Pengetahuan TinggiDocument1 pageChapter 4: Thermochemistry Objective Questions: Pengetahuan TinggiChrisLeeNo ratings yet
- Experimen T Set-Up of Apparatus ObservationDocument1 pageExperimen T Set-Up of Apparatus ObservationChrisLeeNo ratings yet
- By Referring To The Graph, Explain How Auxin Affects The Growth of Shoot and Roots. Shoot: Root: (2 Marks)Document1 pageBy Referring To The Graph, Explain How Auxin Affects The Growth of Shoot and Roots. Shoot: Root: (2 Marks)ChrisLeeNo ratings yet
- Growth Response of Organ To Applied Auxin Root - Shoot ShootDocument1 pageGrowth Response of Organ To Applied Auxin Root - Shoot ShootChrisLeeNo ratings yet
- Diagram 2.1 Shows A Human BrainDocument1 pageDiagram 2.1 Shows A Human BrainChrisLeeNo ratings yet