Professional Documents
Culture Documents
(Chapter 9) Manufactured Subtances in Industry: Ravien Alvin Yoges Chan Wai Hon
Uploaded by
Sharvien Rajamanikam0 ratings0% found this document useful (0 votes)
12 views8 pagesUses of sulphuric acid .
Original Title
manufactured substances in industry
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentUses of sulphuric acid .
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
12 views8 pages(Chapter 9) Manufactured Subtances in Industry: Ravien Alvin Yoges Chan Wai Hon
Uploaded by
Sharvien RajamanikamUses of sulphuric acid .
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 8
(Chapter 9)
Manufactured
subtances in Industry
RAVIEN
ALVIN
YOGES
CHAN WAI HON
Sulphuric Acid (H2SO4)
The most important industrial chemicals.
Main use of sulphuric acid is producing fertilizer.
Sulphonic acid is produced by the reaction between sulphuric
acid and hydrocarbon compound, then, it reacts with sodium
hydroxide to form sodium alkyl sulphonate which is a detergent .
This is also an use of sulphuric acid.
Accumulators need an electrolyte to carry chargers and to react
with positive and negative plates during the charging and
discharging process. In the accumulators, sulphuric acid acts as
the electrolyte. ( electrolyte= substance that ionizes when
dissolved in suitable ionizing solvents)
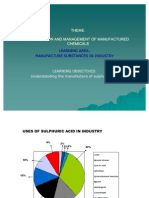
Uses of sulphuric acid
Sales
making fertilizers 38%
detergents 12%
other uses 18%
chemicals 18%
removing rust 1%
paints 13%
Manufacture of sulphuric acid
Three stages in manufacturing sulphuric acid
Stage 1 : Furnance
A) Sulphur is burnt in dry air to produce sulphur dioxide ( S+O2
SO2)
B) In the contact process, sulphur powder is sprayed inside the
furnance at a
temperature of 100c. Here sulphur is converted into sulphur
dioxide.
C) Sulphur dioxide can also be produced by heating metal
sulphate such as Zinc
Sulphate. ( 2ZnS + 3O2
2ZnO + 2SO2 )
Stage 2: The Converter
A) Sulphur dioxide and air are passed over a catalyst called vanadium(V)
oxide, (V2O5).
B) The temperature used here is about (450-500) C . If the temperature is
less than this range, the vanadium(V) oxide may not be ableto catalyse.
C) The reacting pressure is about 2 to 3 atmospheres.
D) At the stage, Sulphur trioxide is produced.(2SO2 + O2
E) This reaction will produce about 98 % sulphur trioxide.
2SO3 )
Stage 3 : the absorber (Formation of sulphuric
acid)
A) Sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum
(H2S2O7) until sulphuric acid reach a concentration of 99.5% (SO3 +
H2SO4
H2S2O7)
B) Oleum will not show any property of acid because it will not ionise without
the presence of water .
C) Water is then added to oleum to produce concentrated sulphuric acid .
(H2S2O7 + H2O
2H2SO4).
D) The reaction a and b is equivalent to dissolving Sulphur trioxide in water.
E) However this reaction is not carried out in industries because its too
vigorous.
Click icon to add picture
You might also like
- Chemistry Manufactured Substances in IndustryDocument81 pagesChemistry Manufactured Substances in Industrynabiellahuda88% (8)
- Hydrostatic and Hydro-Testing in the Oil and Gas FieldFrom EverandHydrostatic and Hydro-Testing in the Oil and Gas FieldRating: 3 out of 5 stars3/5 (2)
- Chapter 2: Sulfur & Sulfuric Acid Chapter 2: Sulfur & Sulfuric AcidDocument20 pagesChapter 2: Sulfur & Sulfuric Acid Chapter 2: Sulfur & Sulfuric AcidabichedNo ratings yet
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresFrom EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresRating: 5 out of 5 stars5/5 (1)
- Practical Synthetic Organic Chemistry: Reactions, Principles, and TechniquesFrom EverandPractical Synthetic Organic Chemistry: Reactions, Principles, and TechniquesNo ratings yet
- DCDADocument23 pagesDCDARushikesh Dhapse80% (5)
- Chemistry Form 4 (Manufactured Substances in Industries)Document24 pagesChemistry Form 4 (Manufactured Substances in Industries)Fariezuan HamidNo ratings yet
- Sulfuric Acid Manufacture: Analysis, Control and OptimizationFrom EverandSulfuric Acid Manufacture: Analysis, Control and OptimizationRating: 3.5 out of 5 stars3.5/5 (3)
- Manufactor Acid SulfuricDocument12 pagesManufactor Acid SulfurictdnguyenNo ratings yet
- Uses of Sulphuric Acid: SulphurDocument6 pagesUses of Sulphuric Acid: SulphurHumphrey JinuinNo ratings yet
- Folio Chemistry: Sulphuric AcidDocument6 pagesFolio Chemistry: Sulphuric AcidmissyunnaNo ratings yet
- Sulphuric AcidDocument3 pagesSulphuric AcidafeequewNo ratings yet
- Sulphuric Acid: The Uses of Sulphuric Acid in Daily LifeDocument2 pagesSulphuric Acid: The Uses of Sulphuric Acid in Daily LifeRos Rusniza Binti SidikNo ratings yet
- Chemistry-Folio Form 4Document45 pagesChemistry-Folio Form 4Ahmad Izzat Mohd HanafiNo ratings yet
- Chemistry Folio: Manufactured Substances in IndustryDocument4 pagesChemistry Folio: Manufactured Substances in IndustryHasnira HazuriNo ratings yet
- Uses of Sulphuric AcidDocument14 pagesUses of Sulphuric AcidFaizul FaiiziNo ratings yet
- Chemistry Form 4 Manufactured Subtances In: Nama ClassDocument7 pagesChemistry Form 4 Manufactured Subtances In: Nama ClassSiti Nur HidayahNo ratings yet
- Chemistry Form 4 Chapter 9 Manufacture Substances in IndustryDocument18 pagesChemistry Form 4 Chapter 9 Manufacture Substances in Industrychulan93100% (15)
- Chemistry Form 4 PDF UPLOADDocument18 pagesChemistry Form 4 PDF UPLOADRahmat Syafiq MuhammadNo ratings yet
- Manufacture of Sulphuric Acid via Contact Process (39 charactersDocument12 pagesManufacture of Sulphuric Acid via Contact Process (39 charactersAdil Yaqub - 74665/TCHR/CNTBNo ratings yet
- Sul Phu Ric Aci DDocument24 pagesSul Phu Ric Aci DBukhari ShafiqNo ratings yet
- Sulphuric Acids: U O S ADocument10 pagesSulphuric Acids: U O S AMuhamad Dzul MuazzemNo ratings yet
- Basic Raw Material For Sulphur Acid ProductionDocument13 pagesBasic Raw Material For Sulphur Acid ProductionGrace Oluchi0% (1)
- Understand Manufacture of Sulphuric Acid and AmmoniaDocument20 pagesUnderstand Manufacture of Sulphuric Acid and AmmoniaKamal AliqNo ratings yet
- Chapter 9 Folio: Manufactured Substances in Industry.: ChemistryDocument15 pagesChapter 9 Folio: Manufactured Substances in Industry.: ChemistryFaizul IzhamNo ratings yet
- Manufactured Substances in IndustryDocument12 pagesManufactured Substances in IndustryAnnuruddin SamsudinNo ratings yet
- Sulfur Dioxide Capture in Sulfuric Acid and Other Products: 20.1. Nickel Extraction OffgasesDocument11 pagesSulfur Dioxide Capture in Sulfuric Acid and Other Products: 20.1. Nickel Extraction OffgasesJadhira RamirezNo ratings yet
- IntroductionDocument10 pagesIntroductionAmith Singh J100% (1)
- Uses of Sulphuric AcidDocument1 pageUses of Sulphuric AcidCyrus JethroNo ratings yet
- Chemistry Form 4 Chapter 9Document23 pagesChemistry Form 4 Chapter 9Pavitran NeymarNo ratings yet
- Chemistry Form 4: Chapter 9 (Manufacture Substances in Industry)Document17 pagesChemistry Form 4: Chapter 9 (Manufacture Substances in Industry)faiz_son96% (73)
- Sulphur: Sulphur: Sources and UsesDocument4 pagesSulphur: Sulphur: Sources and UsesDavies MasumbaNo ratings yet
- Chemical Equilibrium (Part 2) SDocument117 pagesChemical Equilibrium (Part 2) SNur Ainna LiyanaNo ratings yet
- Flow Diagram of Some Industrial ProcessDocument23 pagesFlow Diagram of Some Industrial ProcessAnirudh AchieverNo ratings yet
- Sulphuric AcidDocument4 pagesSulphuric AcidKhai AzNo ratings yet
- Sulphuric Acid and AmmoniaDocument9 pagesSulphuric Acid and Ammoniaash ashNo ratings yet
- New Microsoft Office Word DocumentDocument6 pagesNew Microsoft Office Word Documentkhengarsadiya99No ratings yet
- Chemistry Form 4 Auto SavedDocument21 pagesChemistry Form 4 Auto SavedTharshini ArivananthanNo ratings yet
- Chemistry Form 4 Chapter 9Document24 pagesChemistry Form 4 Chapter 9Raatheys RaoNo ratings yet
- Properties of Sulphuric AcidDocument3 pagesProperties of Sulphuric AcidTian YinNo ratings yet
- Modern Chemical ManufacturerDocument8 pagesModern Chemical ManufacturerUltra Gamer (sishant)No ratings yet
- Folio Chemistry F4 (Manufactured Substances in Industry)Document31 pagesFolio Chemistry F4 (Manufactured Substances in Industry)JackOss93No ratings yet
- AcidDocument2 pagesAcidShahrul NiezamNo ratings yet
- Manufactured Substance in Industry: Nurul Rahwani BT Abdul Rahim Izzah Nur Syazana BT TukiranDocument12 pagesManufactured Substance in Industry: Nurul Rahwani BT Abdul Rahim Izzah Nur Syazana BT TukiranAdam PayneNo ratings yet
- Cambridge IGCSE Chemistry SulferDocument4 pagesCambridge IGCSE Chemistry SulferAmna ImranNo ratings yet
- Kimia Chapter 9Document35 pagesKimia Chapter 9Mohammad AmirNo ratings yet
- Proses SentuhDocument7 pagesProses SentuhSuriati Bt A RashidNo ratings yet
- Sulfuric Acid Obtaining: Ricardo Ocampo SánchezDocument7 pagesSulfuric Acid Obtaining: Ricardo Ocampo SánchezAgueda ZuñigaNo ratings yet
- Organic Reaction Mechanisms 2011: An annual survey covering the literature dated January to December 2011From EverandOrganic Reaction Mechanisms 2011: An annual survey covering the literature dated January to December 2011A. C. KnipeNo ratings yet
- Ligand Platforms in Homogenous Catalytic Reactions with Metals: Practice and Applications for Green Organic TransformationsFrom EverandLigand Platforms in Homogenous Catalytic Reactions with Metals: Practice and Applications for Green Organic TransformationsNo ratings yet