Professional Documents
Culture Documents
Topic 1 Exercise Answers
Topic 1 Exercise Answers
Uploaded by
a08000010660 ratings0% found this document useful (0 votes)
5 views2 pagesThe document contains sample exercises and answers for topics 1 and 1.2 in chemistry. It includes:
1) Exercises on writing electron configurations with the number of protons, neutrons, and electrons specified.
2) Exercises involving drawing electron configurations for elements and ions.
3) An explanation of why successive ionization energies generally increase and why there are sometimes large jumps between some ionization energies.
Original Description:
jj
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains sample exercises and answers for topics 1 and 1.2 in chemistry. It includes:
1) Exercises on writing electron configurations with the number of protons, neutrons, and electrons specified.
2) Exercises involving drawing electron configurations for elements and ions.
3) An explanation of why successive ionization energies generally increase and why there are sometimes large jumps between some ionization energies.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views2 pagesTopic 1 Exercise Answers
Topic 1 Exercise Answers
Uploaded by
a0800001066The document contains sample exercises and answers for topics 1 and 1.2 in chemistry. It includes:
1) Exercises on writing electron configurations with the number of protons, neutrons, and electrons specified.
2) Exercises involving drawing electron configurations for elements and ions.
3) An explanation of why successive ionization energies generally increase and why there are sometimes large jumps between some ionization energies.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 2
Answers to Topic 1 Exercises
1.1.1 Exercise 1
1.
4.
7.
10.
11.
15.
1p, 0n, 1e
54p, 78n, 54e
1p, 0n, 0e
6p, 8n, 6e
39 +
K
12.
127 I
2.
5.
8.
8p, 9n, 8e
13p, 14n, 10e
21p, 24n, 18e
3.
6.
9.
2p, 2n, 0e
92p, 143n, 92e
17p, 20n, 18e
16
O2-
13.
14.
208
Pb2+
107.96
3.
10.85
4.
69.80
1.1.1 Exercise 2
1.
28.29
2.
1.2.1 Exercise 1
1.
2.
3.
4.
1s
2s
1s
2s
1s
2s
2p
[Ne
]
8.
3s
[Ar]
[Ar]
4s
[Ar]4s1
3p
1s22s22p63s23p6
5.
4s
9.
3s
4s
3d
2p
1s22s22p6
7.
10.
2p
11.
6.
1s22s22p63s23p6
3p
3d
3d
[Ar] 4s23d104p3
4p
12.
[Ar] 4s23d104p6
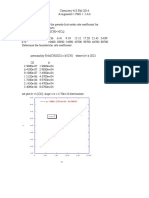
1.2.1 Exercise 2 Successive Ionisation Energies
1. a)
b)
Successive ionisation energies always increase because as an electron is
removed from a shell, the repulsion between the remaining electrons
decreases, as a result of which they move closer to the nucleus and so
more energy is required to remove them
There is a large increase between the 4th and 5th ionisation energies and
between the 11th and 12th ionisation energies because the 5th and 12th
electrons are removed from an inner shell. As a result there is a large drop
in shielding and the remaining electrons are much harder to remove.
2.
You might also like
- Solutions To A) Exercises: Atkins, de Paula & Friedman: Physical Chemistry: Quanta, Matter, and Change 2eDocument30 pagesSolutions To A) Exercises: Atkins, de Paula & Friedman: Physical Chemistry: Quanta, Matter, and Change 2erafelNo ratings yet
- Sci Focus 2 HB AnswersDocument38 pagesSci Focus 2 HB Answersal83rt777760% (5)
- Atkins Solutions (A)Document32 pagesAtkins Solutions (A)baba bababNo ratings yet
- Chapter8 Vapor Cycles ProblemsDocument77 pagesChapter8 Vapor Cycles ProblemsAldren ArnaizNo ratings yet
- Answers To Topic 1 Exercises Topic 1 Exercise 1Document2 pagesAnswers To Topic 1 Exercises Topic 1 Exercise 1hamna rizwanNo ratings yet
- Imp Ques Maths Class 12Document5 pagesImp Ques Maths Class 12CPlanetFdkNo ratings yet
- Ass 1Document3 pagesAss 1vanesaNo ratings yet
- Neet KTG Thermodynamics) - Answer KeyDocument11 pagesNeet KTG Thermodynamics) - Answer KeybalramsharmaNo ratings yet
- NCERT Imp Q.Document2 pagesNCERT Imp Q.Manisha YadavNo ratings yet
- Enrich 1: Electronic Configuration: AnswersDocument3 pagesEnrich 1: Electronic Configuration: AnswersIsh AldayNo ratings yet
- Chemistry of Life Sciences Peter Atkins 10e Answers ExercisesDocument32 pagesChemistry of Life Sciences Peter Atkins 10e Answers ExercisesAnant MadhavNo ratings yet
- Ncert CategorisedDocument9 pagesNcert CategorisedScribdwqNo ratings yet
- Soluzioni CormenDocument20 pagesSoluzioni Cormengiacomo rossiNo ratings yet
- Math RevisionDocument12 pagesMath RevisionAddy The humanNo ratings yet
- Allen: Nurture COURSEDocument8 pagesAllen: Nurture COURSEsiydifaNo ratings yet
- Allen: Nurture COURSEDocument8 pagesAllen: Nurture COURSEsiydifaNo ratings yet
- Pchem11e Student Answers A4Document44 pagesPchem11e Student Answers A4Shanthi GeoNo ratings yet
- Mathematics X ImportanDocument2 pagesMathematics X ImportanSYED BILAL ALINo ratings yet
- Super Education Group: 5.1 The Composition of AirDocument5 pagesSuper Education Group: 5.1 The Composition of AirSgl Edriclai HorngNo ratings yet
- UntitledDocument2,809 pagesUntitledOscar UrielNo ratings yet
- Revision Questions / Problems From H.C. Verma (Part-1) : PhysicsDocument2 pagesRevision Questions / Problems From H.C. Verma (Part-1) : PhysicsOmkar PachoreNo ratings yet
- Part TestDocument11 pagesPart TestamanweshdasNo ratings yet
- Chem 432 Practice Exam #3 Key S18Document6 pagesChem 432 Practice Exam #3 Key S18marksteve160No ratings yet
- Solutions To Problems, Capitulo 2 LevenspielDocument6 pagesSolutions To Problems, Capitulo 2 LevenspielAlexander Gonzalez Romero75% (4)
- Pseudo-Pressure Case For Modified Isochronal Test Without Stabilized PointDocument17 pagesPseudo-Pressure Case For Modified Isochronal Test Without Stabilized PointyerkoNo ratings yet
- EM Assignments Sp19Document2 pagesEM Assignments Sp19Cristian LopezNo ratings yet
- Chemistry and Chemical Reactivity 9th Edition Kotz Solutions Manual DownloadDocument15 pagesChemistry and Chemical Reactivity 9th Edition Kotz Solutions Manual DownloadTodd Dean100% (26)
- STD: XII (Science) Maths IMP QuestionsDocument3 pagesSTD: XII (Science) Maths IMP QuestionsYash PatelNo ratings yet
- 7.1 Answer Keys Major Test 7 15 April 2018 - UnlockedDocument3 pages7.1 Answer Keys Major Test 7 15 April 2018 - UnlockedRahul MishraNo ratings yet
- Chemistry Removed Topics 2021-2022Document1 pageChemistry Removed Topics 2021-2022Aditya ChawlaNo ratings yet
- Answers To Exercises 2014Document7 pagesAnswers To Exercises 2014Ana María Muñoz JaramilloNo ratings yet
- AIATS Medical 2014 Test-9 SolutionDocument7 pagesAIATS Medical 2014 Test-9 Solutionblue_l1No ratings yet
- Answer Key: 1.7. Quadratic InequalityDocument10 pagesAnswer Key: 1.7. Quadratic InequalityPrincess BoloyNo ratings yet
- Answer Key: 1.7. Quadratic InequalityDocument10 pagesAnswer Key: 1.7. Quadratic InequalityPrincess BoloyNo ratings yet
- Neet Key 007Document2 pagesNeet Key 007Jay PatelNo ratings yet
- 10th Maths Imporatant Question English Medium PDF DownloadDocument3 pages10th Maths Imporatant Question English Medium PDF DownloadSvkumar SivaNo ratings yet
- 001.instrumentation 20120911Document11 pages001.instrumentation 20120911Marisa HuangNo ratings yet
- Target: Pre-Medical 2013 Major Test # 02: NEET-UGDocument4 pagesTarget: Pre-Medical 2013 Major Test # 02: NEET-UGsivarajeshwarNo ratings yet
- ThermodynamicsDocument12 pagesThermodynamicsKira ToNo ratings yet
- 6.2 Solution of Initial Value Problems 317Document1 page6.2 Solution of Initial Value Problems 317guardian45No ratings yet
- Solutions - AIATS JEE (Main) - 2016 - Test-8 - (Code-A & B) PDFDocument24 pagesSolutions - AIATS JEE (Main) - 2016 - Test-8 - (Code-A & B) PDFpachuNo ratings yet
- Answer Enhance MergeDocument99 pagesAnswer Enhance MergeAllen RodriguezNo ratings yet
- Themo Tutorials Part 1Document59 pagesThemo Tutorials Part 1Jenae CarlsonNo ratings yet
- Ap Chemistry February: Date Activity Homework Text RefDocument2 pagesAp Chemistry February: Date Activity Homework Text RefDuong NguyenNo ratings yet
- PH 11 CHP 9Document1 pagePH 11 CHP 9Kasim hemdenNo ratings yet
- Aiats Aipmt 2015 Test-2Document9 pagesAiats Aipmt 2015 Test-2Juhi NeogiNo ratings yet
- Allen KeyDocument11 pagesAllen KeyRavi Kiran KoduriNo ratings yet
- Elements of Physical Chemistry 5e SM AnswersDocument22 pagesElements of Physical Chemistry 5e SM Answersherecomesthasun100% (1)
- Distance Learning Programme: Pre-Medical: Leader Test Series / Joint Package CourseDocument4 pagesDistance Learning Programme: Pre-Medical: Leader Test Series / Joint Package Coursepawan paudelNo ratings yet
- Ch. 1-4 Assignments: Will Need Calculator ProgramDocument11 pagesCh. 1-4 Assignments: Will Need Calculator ProgramajramrothNo ratings yet
- Imps NCERT MathsDocument13 pagesImps NCERT MathsShubh ChandegaraNo ratings yet
- Achiever Maza Minor-02 04 June SoDocument6 pagesAchiever Maza Minor-02 04 June SoJeetraj SoniNo ratings yet
- Important Maths QuestionsDocument2 pagesImportant Maths Questionsriteshpandey12842No ratings yet
- REDOX HandoutDocument5 pagesREDOX HandoutKeith Ian QuijalvoNo ratings yet
- Elementary Laplace Transform TableDocument1 pageElementary Laplace Transform TableGustavo XumbinNo ratings yet
- Solution Thermodynamics IntroductionDocument43 pagesSolution Thermodynamics IntroductionDian Remarthin GirsangNo ratings yet
- Simplifying SurdsDocument2 pagesSimplifying SurdsGonzalo Tan Jr.No ratings yet
- Major DLP Test-02 04 Feb QPDocument11 pagesMajor DLP Test-02 04 Feb QPRavi PrakashNo ratings yet
- Model Answers in Ordinary National Certificate Mathematics for EngineersFrom EverandModel Answers in Ordinary National Certificate Mathematics for EngineersNo ratings yet