Professional Documents
Culture Documents
Protein Structure
Protein Structure
Uploaded by
Jennifer HerediaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Protein Structure
Protein Structure
Uploaded by
Jennifer HerediaCopyright:
Available Formats
Protein Structure
Primary Structure
Proteins are made up of polypeptide chains, which are amino acids joined together
with peptide bonds. The unique sequence of amino acids that make up a protein or
polypeptide chain is called the Primary Structure.
Primary Structure: The unique sequence of amino acids that makes up a protein or
polypeptide chain.
Peptide bonds are created by enzyme catalysed condensation reactions and broken
down by enzyme catalysed hydrolysis reactions. Breaking down proteins is important in
many areas of the body, not merely in digestion. For example, in hormone regulation,
cells that are targeted by hormones contain enzymes to break down those hormones. This
stops their effects from being permanent and allows them to be controlled.
Secondary Structure
After synthesis, polypeptide chains are folded or pleated into different shapes, called
their Secondary Structure. Two common examples of secondary structures are Alpha
Helices and Beta Pleated Sheets. Secondary structure is held together by many
Hydrogen bonds, overall giving the shape great stability.
Secondary Structure: The way in which the primary structure of a polypeptide chain
folds.
Tertiary Structure
The final 3D structure of a protein is its Tertiary Structure, which pertains to the
shaping of the secondary structure. This may involve coiling or pleating, often with
straight chains of amino acids in between.
Tertiary Structure: The final 3D structure of a protein, entailing the shaping of a
secondary structure.
Tertiary structure is held together by four different bonds and interactions:
o
Disulphide Bonds - Where two Cysteine amino acids are found together, a
strong double bond (S=S) is formed between the Sulphur atoms within the Cysteine
monomers.
o
Ionic Bonds - If two oppositely charged 'R' groups (+ve and -ve) are found
close to each other, and ionic bond forms between them.
o
Hydrogen Bonds - Your typical everyday Hydrogen bonds.
o
Hydrophobic and Hydrophilic Interactions - Some amino acids may be
hydrophobic while others are hydrophilic. In a water based environment, a globular
protein will orientate itself such that it's hydrophobic parts are towards its centre and its
hydrophilic parts are towards its edges
Tertiary structure can be broken by the action of heat. Increasing the kinetic energy
of protein with a tertiary structure makes it vibrate more, and so the bonds that maintain
its shape (which are mainly weak, non-covalent bonds) will be more likely to break. When
a protein loses its shape in this way it is said to be Denatured. Even when cool the
protein will not (or is highly unlikely to) form its original complex shape.
Proteins with a 3D structure fall
into two main types:
o
Globular - These tend to
form
ball-like
structures
where
hydrophobic parts are towards the
centre and hydrophilic are towards
the edges, which makes them water
soluble. They usually have metabolic
roles,for example: enzymes in all
organisms, plasma proteins and
antibodies in mammals.
o
Fibrous - They proteins
form long fibres and mostly consist of
repeated sequences of amino acids
which are insoluble in water. They
usually have structural roles, such as:
Collagen in bone and cartilage,
Keratin in fingernails and hair.
Quaternary Structure
Some proteins are made up of multiple polypeptide chains, sometimes with an
inorganic component (for example, a haem group in haemoglogin) called a Prosthetic
Group. These proteins will only be able to function if all subunits are present.
Quaternary Structure: The structure formed when two or more polypeptide chains join
together, sometimes with an inorganic component, to form a protein.
Haemoglobin and Collagen
Haemoglobin is a water soluble globular protein which is composed of two
polypeptide chains, two polypeptide chains and an inorganic prosthetic haem group. Its
function is to carry oxygen around in the blood, and it is facilitated in doing so by the
presence of the haem group which contains a Fe2+ ion, onto which the oxygen molecules
can bind.
Collagen is a fibrous protein consisting of three polypeptide chains wound around
each other. Each of the three chains is a coil itself. Hydrogen bonds form between these
coils, which are around 1000 amino acids in length, which gives the structure strength.
This is important given collagen's role, as structural protein. This strength is increased by
the fact that collagen molecules form further chains with other collagen molecules and
form Covalent Cross Links with each other, which are staggered along the molecules to
further increase stability. Collagen molecules wrapped around each other form Collagen
Fibrils which themselves form Collagen Fibres.
Collagen has many functions:
o
Form the structure of bones
o
Makes up cartilage and connective tissue
o
o
o
o
o
o
Prevents blood that is being pumped at high pressure from bursting the walls
of arteries
Is the main component of tendons, which connect skeletal muscles to bones
Haemoglobin may be compared with Collagen as such:
Basic Shape - Haemoglobin is globular while Collagen is fibrous
Solubility - Haemoglobin is soluble in water while Collagen is insoluble
Amino Acid Constituents - Haemoglobin contains a wide range of amino
acids while Collagen has 35% of it primary structure made up of Glycine
Prosthetic Group - Haemoglobin contains a haem prosthetic group while
Collagen doesn't possess a prosthetic group
Tertiary Structure - Much of the Haemoglobin molecule is wound into
helices while much of the Collagen molecule is made up of left handed helix structures
You might also like
- Microbiological Quality of Palm Kernel FruitsDocument6 pagesMicrobiological Quality of Palm Kernel FruitsPrecious NwankwoNo ratings yet
- Biology Chapter 1 STPM Sem1Document12 pagesBiology Chapter 1 STPM Sem1Jia Hui100% (4)
- ACFrOgBIo4EBT4MUcI4imTruT1aNJ6WpW10DkogaFN4PE9HmJXc Y 60 DkfXsb6z5psCLCyW5dQPe0KnxUiH7kkbYTgNdfTMDrseYL6qC z13btWzKkhHM1sg Pi8GyycDMgWD FIafmagyLtfDocument136 pagesACFrOgBIo4EBT4MUcI4imTruT1aNJ6WpW10DkogaFN4PE9HmJXc Y 60 DkfXsb6z5psCLCyW5dQPe0KnxUiH7kkbYTgNdfTMDrseYL6qC z13btWzKkhHM1sg Pi8GyycDMgWD FIafmagyLtfbody fayezNo ratings yet
- SOM 201 Nutrients Notes 2021Document26 pagesSOM 201 Nutrients Notes 2021kxng crocked100% (1)
- D.Pharma 1st Year Biochemistry and Clinical Pathology Ebook (Udit Pharmacy) by Udit Narayan VishwakarmaDocument59 pagesD.Pharma 1st Year Biochemistry and Clinical Pathology Ebook (Udit Pharmacy) by Udit Narayan Vishwakarmajaswinder singh100% (4)
- Protein Structure WorksheetDocument7 pagesProtein Structure WorksheetkarendbrooksNo ratings yet
- ProteinsDocument9 pagesProteinsJada HartNo ratings yet
- Protein: Globular and Fibrous ProteinDocument20 pagesProtein: Globular and Fibrous ProteinAndrewNo ratings yet
- Proteins: Classification FunctionsDocument20 pagesProteins: Classification FunctionsGeo NalobNo ratings yet
- Proteins: NH (Amine) Group COOH (Carboxylic) GroupDocument5 pagesProteins: NH (Amine) Group COOH (Carboxylic) GroupPriyanthi FernandoNo ratings yet
- 1 LGDocument51 pages1 LGJoo Se HyukNo ratings yet
- L7 - Protein StructureDocument29 pagesL7 - Protein Structureemaniftikhar56No ratings yet
- Protein AsDocument46 pagesProtein AsYousef WardatNo ratings yet
- Jeremiah Whyte: Biology AssignmentDocument5 pagesJeremiah Whyte: Biology AssignmentJeremiah WhyteNo ratings yet
- Chapter 4 Lecture PptsDocument82 pagesChapter 4 Lecture PptsJota AlcuadradoNo ratings yet
- Structure of ProteinsDocument3 pagesStructure of ProteinsRam Kewal TripathiNo ratings yet
- ProteinsDocument20 pagesProteinsGrishma RanaNo ratings yet
- BiochemistryDocument130 pagesBiochemistryshiyntumNo ratings yet
- 4 ProteinsDocument8 pages4 ProteinscarnevermelhaNo ratings yet
- Class Notes - Biology SAC 1Document24 pagesClass Notes - Biology SAC 1carmelynnnamoresNo ratings yet
- Bion Peptides and Proteins First Semester 2021 2022Document55 pagesBion Peptides and Proteins First Semester 2021 2022Hashem Bani yaseenNo ratings yet
- Level of Organisation of Protein StructureDocument18 pagesLevel of Organisation of Protein Structureyinghui94No ratings yet
- Primary, Secondary, Tertiary and Quaternary Structures of A ProteinDocument21 pagesPrimary, Secondary, Tertiary and Quaternary Structures of A ProteinDALITSO CHIKOYA100% (1)
- Lecture 3-ProteinsDocument9 pagesLecture 3-ProteinsOminousCroakNo ratings yet
- Structures of Proteins: Objective 1.8 Presented by Group 4Document12 pagesStructures of Proteins: Objective 1.8 Presented by Group 4Helpful HandNo ratings yet
- Biomolecules: MacromoleculesDocument5 pagesBiomolecules: MacromoleculesPaulaNo ratings yet
- Proteins - Many Structures, Many FunctionsDocument30 pagesProteins - Many Structures, Many FunctionsXEDGER09No ratings yet
- Biology RevisionDocument2 pagesBiology RevisionamarsodhaNo ratings yet
- Biological Molecules Full Notes SummaryDocument10 pagesBiological Molecules Full Notes SummaryHaffee FaizalNo ratings yet
- Lecture - ProteinsDocument62 pagesLecture - ProteinsBahesty Monfared AkashNo ratings yet
- 3-Bch303 Chapter3 Protein Structure and FunctionDocument105 pages3-Bch303 Chapter3 Protein Structure and Functionsandaramae04No ratings yet
- Chapter 4 Cell BioDocument22 pagesChapter 4 Cell BioJennyNo ratings yet
- البروتيناتDocument54 pagesالبروتيناتYaman HassanNo ratings yet
- As 2 Biological MoleculesDocument29 pagesAs 2 Biological MoleculesisaacNo ratings yet
- 4 - Protein StructureDocument30 pages4 - Protein StructureInnocent TebuloNo ratings yet
- Lecture 2 - BiomoleculesDocument55 pagesLecture 2 - BiomoleculesRamkiNo ratings yet
- IndustrialDocument18 pagesIndustrialAman KhanNo ratings yet
- Different Properties of WaterDocument4 pagesDifferent Properties of WaterKassandra Bravo SagunNo ratings yet
- Secondary (2º) StructureDocument13 pagesSecondary (2º) Structurebenina biancaNo ratings yet
- 1.4 ProteinDocument55 pages1.4 ProteinCally ChanNo ratings yet
- Chemistry of Protein IIDocument36 pagesChemistry of Protein IIatfbdalmsyh672No ratings yet
- 1A 5: Proteins: Ems School As Biology Shameelah R. BalkhiDocument34 pages1A 5: Proteins: Ems School As Biology Shameelah R. Balkhimuhammad naufalNo ratings yet
- Structure of ProteinsDocument9 pagesStructure of ProteinsNagina naginaNo ratings yet
- Proteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDocument12 pagesProteins Include A Diversity of Structures, Resulting in A Wide Range of FunctionsDanielle GuerraNo ratings yet
- AFN 3209 - Protein StructureDocument8 pagesAFN 3209 - Protein StructureieunicemuthengiNo ratings yet
- Biological Molecules AS LevelDocument16 pagesBiological Molecules AS LevelAmal ZahraNo ratings yet
- Unit 2 - Enzymes and The Digestive SystemDocument11 pagesUnit 2 - Enzymes and The Digestive SystemKatherine NunnNo ratings yet
- A Level Notes UpdatedDocument19 pagesA Level Notes UpdatedRayonesh RayanaNo ratings yet
- 02 BCH101 Lecture 2 ProteinDocument37 pages02 BCH101 Lecture 2 Proteinsharkar1059No ratings yet
- Topic-1A (Food and Health) (Autosaved) - 60-80Document21 pagesTopic-1A (Food and Health) (Autosaved) - 60-80lisaNo ratings yet
- Proteinsyr12 111011115041 Phpapp01Document22 pagesProteinsyr12 111011115041 Phpapp01safa_sabaNo ratings yet
- BiochemDocument16 pagesBiochemRam RamNo ratings yet
- ProteinsDocument22 pagesProteinsassmmNo ratings yet
- Structures of ProteinsDocument15 pagesStructures of ProteinsEdward Juanico HerradaNo ratings yet
- Proteins: Proteins Are Large Biomolecules, or Macromolecules, Consisting of One orDocument15 pagesProteins: Proteins Are Large Biomolecules, or Macromolecules, Consisting of One orKaleem KhanNo ratings yet
- Structure of ProteinsDocument4 pagesStructure of ProteinsDerick SelvaNo ratings yet
- 05-Protein Structure and FunctionDocument41 pages05-Protein Structure and Functionصدام حسینNo ratings yet
- Protien ASDocument25 pagesProtien ASRabia RafiqueNo ratings yet
- Quaternary StructureDocument2 pagesQuaternary StructureJane ConstantinoNo ratings yet
- ProteinDocument23 pagesProteinsamantha garciaNo ratings yet
- Protein Structure and FunctionDocument30 pagesProtein Structure and Functionمحمد جانNo ratings yet
- What Is A ProteinDocument2 pagesWhat Is A ProteinCoca CollaNo ratings yet
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Worksheet On DNA and RNA AnswersDocument3 pagesWorksheet On DNA and RNA AnswersSelena CoronelNo ratings yet
- Chemistry and Fundamental BiotechnologyDocument3 pagesChemistry and Fundamental BiotechnologyRaweeha SaifNo ratings yet
- Important EnzymesDocument15 pagesImportant EnzymesErin HillNo ratings yet
- Neuropathy Medicines PDFDocument3 pagesNeuropathy Medicines PDFmailbabuNo ratings yet
- Human Genetics 10th Edition Lewis Test BankDocument21 pagesHuman Genetics 10th Edition Lewis Test Bankhubertbak126100% (32)
- Grade 10 ScienceDocument7 pagesGrade 10 ScienceChristian jade QuijanoNo ratings yet
- Hydropathic PlotsDocument14 pagesHydropathic PlotsKrithika Balasubramanian100% (2)
- Nucleic Acid: Group 2 ReportDocument20 pagesNucleic Acid: Group 2 ReportDiePalAPieNo ratings yet
- Unit 2 Biological MoleculesDocument34 pagesUnit 2 Biological MoleculesEbenezer AbrahamNo ratings yet
- BioChem LecDocument21 pagesBioChem LecAbby Dimalaluan OquendoNo ratings yet
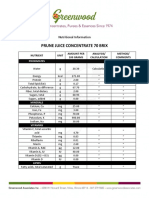
- Prune Juice Concentrate 70 Brix Nutritional InformationDocument2 pagesPrune Juice Concentrate 70 Brix Nutritional Informationborn2dive 9702No ratings yet
- Worksheet - Dna Protein SynthesisDocument2 pagesWorksheet - Dna Protein Synthesisapi-270403367100% (1)
- Eicosanoids: (Prostaglandins, Thromboxanes, Leukotrienes)Document26 pagesEicosanoids: (Prostaglandins, Thromboxanes, Leukotrienes)BOsch VakilNo ratings yet
- Biochemistry 4Th Edition Voet Test Bank Full Chapter PDFDocument46 pagesBiochemistry 4Th Edition Voet Test Bank Full Chapter PDFciaramilcahbrpe100% (14)
- Lesson Plan in Chemistry - PasaDocument6 pagesLesson Plan in Chemistry - PasaPenelope Soria EjadaNo ratings yet
- 3 ProteinsDocument42 pages3 ProteinsTeam BEENo ratings yet
- Performance Task On Nucleic AcidsDocument3 pagesPerformance Task On Nucleic AcidsPrincess Mejia De VeraNo ratings yet
- Difference Between DNA and RNA: February 2017Document10 pagesDifference Between DNA and RNA: February 2017JihanNo ratings yet
- St. Thomas Academy: Pob. 3, City of Sto. Tomas, Batangas SY 2021 - 2022Document4 pagesSt. Thomas Academy: Pob. 3, City of Sto. Tomas, Batangas SY 2021 - 2022Kate Ashly Mae AteradoNo ratings yet
- Tutorial Chapter 4 BioDocument9 pagesTutorial Chapter 4 BioZunnurain AmniNo ratings yet
- St. Thomas Academy: Part A. Classify Each As A Carbohydrate, Protein, Lipid or Nucleic Acid (Only Used Once)Document2 pagesSt. Thomas Academy: Part A. Classify Each As A Carbohydrate, Protein, Lipid or Nucleic Acid (Only Used Once)Kate Ashly Mae AteradoNo ratings yet
- 9protein EngineeringhandoutDocument21 pages9protein EngineeringhandoutSimon Uribe PNo ratings yet
- Cobb500 BPN Supp 2008 (LBS)Document6 pagesCobb500 BPN Supp 2008 (LBS)Umar Iqbal100% (1)
- CSEC - Nutrition Powerpoint Scribd VERSIONDocument20 pagesCSEC - Nutrition Powerpoint Scribd VERSIONC ROBERTSNo ratings yet
- Biochemistry Module 7 & 8Document2 pagesBiochemistry Module 7 & 8Tricia Ann A. SodsodNo ratings yet
- JELS Vol 2 No 2 2012 PDFDocument57 pagesJELS Vol 2 No 2 2012 PDFErika ViyanaNo ratings yet
- Noon Products Product List 2023 11-30-10 02Document11 pagesNoon Products Product List 2023 11-30-10 02Shabeeh AliNo ratings yet