Professional Documents
Culture Documents
Multielectron Atoms PDF
Multielectron Atoms PDF
Uploaded by
guruOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Multielectron Atoms PDF
Multielectron Atoms PDF
Uploaded by
guruCopyright:
Available Formats
Atomic Term Symbols
From a spectroscopic perspective, we need to know the values
for the various types of angular momenta. Term symbols
provide three pieces of information
1. Total orbital angular momentum, L
2. Multiplicity of the term, 2S+1
3. Total angular momentum, J
2 S+1
LJ
Atomic Term Symbols
v
L Total
v
L2
v
L1
Imagine then summing these vectors together. We can do it,
but it is far easier to consider z-components as these add
together as scalars!
Atomic Term Symbols
In terms of z-components
Ml = ml1 + ml 2 + ml3 + ....
Or in terms of magnitudes
L = l1 + l 2 + l 3 + ....
The total # or series of possible values for all orientations is
given by the Clebsch-Gordon Series. For an atom with
two electrons,
L = l 1 + l 2 , l 1 + l 2 1, l 1 + l 2 2,... l 1 l 2
Atomic Term Symbols
L = l 1 + l 2 , l 1 + l 2 1, l 1 + l 2 2,... l 1 l 2
Maximum L corresponds to
l1 + l 2
Minimum L corresponds to
l1 l 2
Quantum Number L designations are
L:
0
S
1
P
2
D
3
F
4
G
Atomic Term Symbols
How does this work then for an atom with an electron
configuration with np2 (e.g., 1s2,2s22p2 or
1s22s22p63s23p2)?
L = l1 + l 2 , l1 + l 2 1, l1 + l 2 2,...l1 l 2
L = 2, 1, 0
Thus potential terms are D, P, S and each of these
terms will differ in energy.
Atomic Term Symbols
Multiplicity
Can also determine the multiplicity for the term using a
Clebsch-Gordon series
S=+,-
= 1, 0
for two electron system
Multiplicity given by 2S + 1
For S =1: 2S+1 = 3
For S=0: 2S+1 = 1
Called a triplet state
Called a singlet state
Atomic Term Symbols
Total Angular Momentum
Permitted values of J again, given by a Clebsch-Gordon series
J = L + S, L + S - 1, L + S - 2 . |L - S|
Atomic Term Symbols
As a first example, lets consider the hydrogen atom
For case with L = 1, S =
term symbols are
2P
3/2
J = 1 + and 1 -
and 2P1/2
Atomic Term Symbols
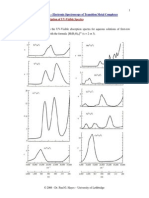
A portion of the hydrogen atom transition level diagram for
optical spectra then, will look like
2S
1/2
3s
2P
3/2
2P
1/2
2P
3/2
2S
1/2
2S
1/2
2s
1s
2P
1/2
3p
3p
2p
2p
2D
5/2
2D
3d
3/2
}spin-orbit
3d
coupling
Selection rules for optical
transitions
J = 0,1
l = 1
s = 0
Atomic Term Symbols
A portion of the hydrogen atom transition level diagram for
optical spectra then, will look like
2S
1/2
3s
2P
3/2
2P
1/2
2P
3/2
2S
1/2
2S
1/2
2s
1s
2P
1/2
3p
3p
2p
2p
2D
5/2
2D
3d
3/2
}spin-orbit
3d
coupling
Selection rules for optical
transitions
J = 0,1
l = 1
s = 0
Atomic Term Symbols
Lets look at some other examples. What is the term for the
ground state of helium? (1s2)
Here
L=0 (why?)
S=0 (why?)
Term then is 1So.
Atomic Term Symbols
What about Ne?
For 2p6 configuration, only one set of possible values.
ML= m1 + m2 + m3 + m4 + m5 + m6
= 1 + 1 + 0 + 0 + (-1) + (-1) = 0
And in this case we also have
S = |Ms| = ms1 + ms2 + ms3 + ms4 + ms5 + ms6
= - + - + - =0
Thus, term is 1So. We will find this to be true for ANY filled
subshell. More interesting then to consider unfilled shells.
Atomic Term Symbols
What is the term symbol for helium with a 1s12s1
configuration?
L = 0 (why?)
S = + , - = 1, 0
Thus there are two possibilities: 3S and 1S
Atomic Term Symbols
Energy level diagram for helium then is
parahelium
1S
1P
1D
orthohelium
3S
3P
3D
Atomic Term Symbols
What about an atom with the configuration 1s2,2s2, 2p1,3p1
(corresponds to an excited state for carbon)
L = l1 + l 2 , l1 + l 2 1, l1 + l 2 2,...l1 l 2
L = 2, 1, 0
D, P, and S
S can have S = 1, 0
Given these values for L and S we have
3D, 1D, 3P, 1P, 3S,
and 1S
Atomic Term Symbols
Including total orbital angular momentum, we need to consider
the following
3D
3,
3D
2,
3D
1,
1D
2,
3P , 3P , 3P , 1P , 3S ,
2
1
0
1
1
and 1So
Question is, which of these terms corresponds to the lowest
energy state??
Use Hunds Rules to determine!
Atomic Term Symbols
Hunds Rules
1. State with the largest value of S is most stable and stability
decreases with decreasing S.
2. For states with same values of S, the state with the largest
value of L is the most stable.
3. If states have same values of L and S then, for a subshell
that is less than half filled, state with smallest J is most
stable; for subshells that are more than half filled, state with
largest value of J is most stable.
Atomic Term Symbols
Here we are considering the terms 3D, 3P, 3S, 1D, 1P, 1S. In
terms of stability we can rank these terms as
1S
1P
1D
3S
3P
3D
Most stable
Given that the 3D states are most stable, which of these terms
correspond to the most stable state?
Atomic Term Symbols
Since the two p subshells are less than half filled, we would
predict that the 3D1 term corresponds to the most stable
state!
Simple approach for finding the ground state term symbol for
any atom:
1. Find maximum value of S consistent with the Pauli
Exclusion Principle S = Smax.
2. For S = Smax, find the maximum value of L consistent with
the Pauli Exclusion Principle L = Lmax.
3. Apply Hunds Rules to find J for most stable state.
You might also like
- ASTM D4253-16e1Document14 pagesASTM D4253-16e1katherine Melo100% (1)
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0715-0765)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0715-0765)Administracion OTIC IVICNo ratings yet
- Organic Chemistry Cambridge As-Level SummaryDocument4 pagesOrganic Chemistry Cambridge As-Level SummaryReynaldi BozzNo ratings yet
- The Electronic Spectra of Coordination CompoundsDocument52 pagesThe Electronic Spectra of Coordination CompoundsAyuditNo ratings yet
- Bacteria QuizDocument10 pagesBacteria QuizMark Abion ValladolidNo ratings yet
- Atom Levels Term SymbolDocument21 pagesAtom Levels Term SymbolUlfa WulandariNo ratings yet
- Electronic SpectraDocument22 pagesElectronic SpectraVishnu ChariNo ratings yet
- Electronic Spectroscopy 1Document62 pagesElectronic Spectroscopy 1api-372459780% (5)
- Transition Metals and Coordination CompoundsDocument54 pagesTransition Metals and Coordination CompoundsCaryl FrancheteNo ratings yet
- CHEM20024 Lecture Notes 11 - Free Energy and EquilibriumDocument27 pagesCHEM20024 Lecture Notes 11 - Free Energy and EquilibriumEzriel QuantumNo ratings yet
- ATOICV1 8 9 Charge Transfer Spectra NawazDocument16 pagesATOICV1 8 9 Charge Transfer Spectra Nawazsaira mehmoodNo ratings yet
- Silverstein - Spectrometric Identification of Organic Compounds 7th EdDocument550 pagesSilverstein - Spectrometric Identification of Organic Compounds 7th EdKarla LopesNo ratings yet
- Metal Ligand Bonding PDFDocument41 pagesMetal Ligand Bonding PDFSPEAK YOUR HEART OUTNo ratings yet
- Migratory InsertionDocument13 pagesMigratory InsertionNitaEkawatiNo ratings yet
- Aromaticity, Antiaromaticity, Homoaromaticity and The Hückel (4n + 2) RuleDocument15 pagesAromaticity, Antiaromaticity, Homoaromaticity and The Hückel (4n + 2) RuleKishore KishoreNo ratings yet
- LCAO MO Theory Illustrated by Its Application To H2Document8 pagesLCAO MO Theory Illustrated by Its Application To H2maugonzalezsuarezNo ratings yet
- 1 IR NMR Practice ProblemsetDocument12 pages1 IR NMR Practice ProblemsetJustin BuiNo ratings yet
- Orgel Diagram: Prof. Robert J. LancashireDocument5 pagesOrgel Diagram: Prof. Robert J. LancashireMa'arif A. Syafii0% (1)
- Electron Transfer Redox ReactionsDocument33 pagesElectron Transfer Redox ReactionsAlifiya DholkawalaNo ratings yet
- Silverstein Chapter 1 Mass SpectrometryDocument71 pagesSilverstein Chapter 1 Mass SpectrometryNikita GroverNo ratings yet
- Exp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)Document19 pagesExp 3 (Prep - of Na2S2O3.5H2O) & 4 (Excercise)KarzanNo ratings yet
- Pearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesDocument5 pagesPearson's Classification of Lewis Acids and Lewis Bases Into Hard and Soft - Acids and BasesThantea ChhakchhuakNo ratings yet
- ElectrochemistryDocument215 pagesElectrochemistryvisalNo ratings yet
- Reductive EliminationDocument14 pagesReductive EliminationAɞdȗl NąvêêdNo ratings yet
- Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Document51 pagesPeter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0766-0816)Administracion OTIC IVICNo ratings yet
- ORD CD AMU NotesDocument32 pagesORD CD AMU NotesTil Sen100% (1)
- Nuclear Magnetic Resonance of ProtonsDocument43 pagesNuclear Magnetic Resonance of ProtonsJohnathan InarrittuNo ratings yet
- Preparatory Problems PDFDocument2 pagesPreparatory Problems PDFGerel BayrmagnaiNo ratings yet
- Magnetic Properties of Transition Metal Metal ComplexesDocument9 pagesMagnetic Properties of Transition Metal Metal ComplexestharanginiNo ratings yet
- P-Block Elements (Halogens and Noble Gases) : ChemistryDocument23 pagesP-Block Elements (Halogens and Noble Gases) : ChemistrythinkiitNo ratings yet
- Molecular Term SymbolsDocument3 pagesMolecular Term SymbolsRojo JohnNo ratings yet
- Chemoselectivity Regioselectivity Stereoselectivity: (Which) (Where) (How)Document18 pagesChemoselectivity Regioselectivity Stereoselectivity: (Which) (Where) (How)Akhil PratapNo ratings yet
- Structure of FerroceneDocument5 pagesStructure of FerroceneFelipe Rojas AcevedoNo ratings yet
- ASA PPT NOESYDocument15 pagesASA PPT NOESYgovind ashokrao100% (1)
- TautomerismDocument2 pagesTautomerismZIdanNo ratings yet
- Thermodynamic and Kinetic Aspects of Metal ComplexesDocument27 pagesThermodynamic and Kinetic Aspects of Metal ComplexesWiratama NugrohoNo ratings yet
- 2.11 Term SymbolsDocument3 pages2.11 Term SymbolsRudra Narayana JenaNo ratings yet
- Multi-Electron AtomsDocument8 pagesMulti-Electron AtomsBarbara Barboza LinoNo ratings yet
- Handout For Russell-Saunders Coupling PDFDocument12 pagesHandout For Russell-Saunders Coupling PDFAditiNo ratings yet
- Term SymbolDocument12 pagesTerm SymbolVhie TriastianiNo ratings yet
- 12 Term Symbols and Selection Rules 12.1 The Total Orbital Angular MomentumDocument4 pages12 Term Symbols and Selection Rules 12.1 The Total Orbital Angular MomentumIqbal Aljabir PujionoNo ratings yet
- UVVis SessionDocument64 pagesUVVis SessionLusi LusianaNo ratings yet
- Lecture-12 - 16-11-22Document20 pagesLecture-12 - 16-11-22Alkit SharmaNo ratings yet
- Unit 5 Coordination Chemistry: Graduate Center Inorganic Chemistry (Fall 2012)Document39 pagesUnit 5 Coordination Chemistry: Graduate Center Inorganic Chemistry (Fall 2012)Carlos Cesar Lopez SuarezNo ratings yet
- Spectra El KompleksDocument36 pagesSpectra El KompleksIndryaHidayatiNo ratings yet
- Interpretasi Spektra Elektronik Senyawa Kompleks 1Document33 pagesInterpretasi Spektra Elektronik Senyawa Kompleks 1AsrelNo ratings yet
- Atomic Term SymbolsDocument13 pagesAtomic Term SymbolsAnish RaoNo ratings yet
- Electronic Spectra of ComplexesDocument82 pagesElectronic Spectra of Complexesirembasar2000No ratings yet
- Atomic Term SymbolsDocument4 pagesAtomic Term SymbolsM IkhsanNo ratings yet
- Molecular Term SymbolsDocument5 pagesMolecular Term Symbols1jerushaNo ratings yet
- 6 Electronic SpectroscopyvDocument19 pages6 Electronic SpectroscopyvKeri Gobin SamarooNo ratings yet
- Electronic (Part 1) Lecture 2Document5 pagesElectronic (Part 1) Lecture 2Maryem MostafaNo ratings yet
- Physical Chemistry II: Quantum Chemistry Lecture 19:many-Electron Atoms & Atomic Term SymbolsDocument42 pagesPhysical Chemistry II: Quantum Chemistry Lecture 19:many-Electron Atoms & Atomic Term SymbolsBo-Ji PengNo ratings yet
- CH 14. Electronic Spectros PDFDocument9 pagesCH 14. Electronic Spectros PDFiq3pevicNo ratings yet
- Electronic SpectraDocument17 pagesElectronic SpectrafahmiNo ratings yet
- Chapter 7 Transition Metal ComplexesDocument48 pagesChapter 7 Transition Metal ComplexesrabiaNo ratings yet
- B.Sc. SEM-VI Us06Cche22 Inorganic Chemistry Unit-3 (A) Term Symbol (B) Electronic Spectra of Metal ComplexesDocument34 pagesB.Sc. SEM-VI Us06Cche22 Inorganic Chemistry Unit-3 (A) Term Symbol (B) Electronic Spectra of Metal ComplexesChaithraMallu100% (1)
- Electronic (Part 1) Lecture 2Document11 pagesElectronic (Part 1) Lecture 2Maryem MostafaNo ratings yet
- For Homework AssignmentDocument48 pagesFor Homework AssignmentyinglvNo ratings yet
- Termsymbols PDFDocument4 pagesTermsymbols PDFIqbal Aljabir PujionoNo ratings yet
- Lecture 6Document7 pagesLecture 6ankara271828No ratings yet
- Atomic Term SymbolsDocument13 pagesAtomic Term SymbolsLucsy ThelyanaNo ratings yet
- كيمياء رابع علمي متميزينDocument132 pagesكيمياء رابع علمي متميزينalmuhndsNo ratings yet
- BOEHMER Split-Body Ball Valves PDFDocument24 pagesBOEHMER Split-Body Ball Valves PDFLuka BornaNo ratings yet
- Elpress Catalog 2011Document149 pagesElpress Catalog 2011Mindaugas MockusNo ratings yet
- A. B. C. D.: AnswerDocument56 pagesA. B. C. D.: AnswerSaidNo ratings yet
- Finite Difference Heat Transfer Analyses in ExcelDocument6 pagesFinite Difference Heat Transfer Analyses in ExcelMariano NathanaelNo ratings yet
- MLP ElectrochemistryDocument17 pagesMLP ElectrochemistrySneha GuptaNo ratings yet
- ECE 410 Homework 5 - Solutions Spring 2008: Problem 1Document4 pagesECE 410 Homework 5 - Solutions Spring 2008: Problem 1Ubaid KhanNo ratings yet
- Recyclingof Waste Coconut Shellsas Substitutefor Aggregatesin Mix Proportioningof Concrete Hollow BlocksDocument18 pagesRecyclingof Waste Coconut Shellsas Substitutefor Aggregatesin Mix Proportioningof Concrete Hollow BlocksJohn Denver CuisonNo ratings yet
- 22-Article Text-40-3-10-20210527Document5 pages22-Article Text-40-3-10-20210527Wahyu DwilestariNo ratings yet
- Synthesis of Separation TrainsDocument22 pagesSynthesis of Separation Trains伟铭No ratings yet
- Biodegradable ProductsDocument7 pagesBiodegradable ProductsCorina PopaNo ratings yet
- Topic 1 - Tuned Mass DamperDocument45 pagesTopic 1 - Tuned Mass DamperdhanarajNo ratings yet
- Origins of LifeDocument112 pagesOrigins of LifeDouglas Carvalho100% (2)
- 07 Chapter 7 Wave TransformationDocument27 pages07 Chapter 7 Wave TransformationLava SatNo ratings yet
- Practica de VectoresDocument30 pagesPractica de VectoresIvanLozanoRuizNo ratings yet
- Life Cycle CostDocument64 pagesLife Cycle Costvishwas gaurNo ratings yet
- Flash Separator Design Conversion of Low PDFDocument19 pagesFlash Separator Design Conversion of Low PDFShoaib JadoonNo ratings yet
- Rueegg 1996Document5 pagesRueegg 1996Alexis Pool Zúñiga HuertasNo ratings yet
- Design of Ultra High Performance Concrete As An Overlay in Pavements and Bridge DecksDocument127 pagesDesign of Ultra High Performance Concrete As An Overlay in Pavements and Bridge DecksHendri Hadisi100% (1)
- Applications of Computational Fluid Dynamics in HydraulicsDocument26 pagesApplications of Computational Fluid Dynamics in HydraulicsAditya KaranNo ratings yet
- SPL DetailsDocument6 pagesSPL DetailsPrakash WarrierNo ratings yet
- EXP10 ChromatographyDocument27 pagesEXP10 ChromatographyKiara Sy0% (1)
- Wilson Hardness Conversion Chart - ShortDocument1 pageWilson Hardness Conversion Chart - ShortwellbeingempireNo ratings yet
- Moisture Guide Plastics enDocument22 pagesMoisture Guide Plastics enMaueeMalicdemNo ratings yet
- Neftalí Rojas - Tega - Pulp LifterDocument24 pagesNeftalí Rojas - Tega - Pulp LiftereduardoslmNo ratings yet
- Syllabus - PH 101Document2 pagesSyllabus - PH 101Dr. Pradeep Kumar SharmaNo ratings yet
- Science 6: ND STDocument4 pagesScience 6: ND STCristaLeengaiAclaoNo ratings yet
- Development of A Solid Phase Microextraction Protocol For The GC-MS Citral DegradationDocument8 pagesDevelopment of A Solid Phase Microextraction Protocol For The GC-MS Citral DegradationcarlosNo ratings yet