Professional Documents
Culture Documents
FMEA Training Guide
FMEA Training Guide
Uploaded by
刘朝阳Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
FMEA Training Guide
FMEA Training Guide
Uploaded by
刘朝阳Copyright:
Available Formats
Manufacturing Technology Committee Risk Management Working Group
Risk Management Training Guides
Failure Modes and Effects Analysis Guide
1 Overview
Failure Modes and Effects Analysis (FMEA) is commonly used in a variety of industries for
Risk Management, where simple quantification of risk is insufficient, and where
identification of root causes of risks and means of mitigation are paramount. FMEA is one
of the most useful and effective tools for developing designs, processes and services. The
goal of FMEA is to align the risks as closely as possible with its source. This enables the
determination of the root cause of the risk, and allows the selection of ways to detect the
occurrence of a particular failure and/or to find options to prevent or mitigate the effects of a
particular failure. Good FMEA methodology allows for the identification and documentation
of potential failures of a system and their resulting effects. It also allows for the assessment
of the potential failure to determine actions that would reduce severity, reduce occurrence,
and increase detection.
During FMEA, all steps are analyzed for potential failure opportunities; the ultimate effect
to product quality or patient safety and/or efficacy as a result of each potential failure
opportunity is then quantified; and then adjusted based on capabilities to detect or mitigate,
to reach a final assigned score of risk.. The risk for each failure is often times entered into a
risk score matrix which enables easy determination of the priority and/or level of attention
required to be applied to each step based on its total risk priority number (RPN). The
outcome of the FMEA is a list of recommendations to reduce overall risk to an acceptable
level, and can be used as a source for designing a control strategy.
This document presents guiding principles for the execution of Failure Modes and Effects
Analysis (FMEA). Successful application of any risk management model requires that the
tools are used in concert with an overall quality risk management process, similar to that
described by ICH Q9.
1.1 Use
Failure Modes and Effects Analysis can be a useful tool in:
selection and optimization of drug product formulation
defining the design space for manufacturing processes for drug substances and
drug products
design and manufacture of devices
Training Guide: Failure Modes and Effects Analysis Guide
A list of advantages and disadvantages of the FMEA tool is provided as follow:
Advantages
Disadvantages
- Accepts a high degree of complexity. - Requires significant effort in
establishing clearly defined terms.
- Uniform quantification of risk can be
applied.
- Requires significant effort in assigning
scores to each step.
- Results can be correlated directly with
actual risks.
- The effect of various methods of
mitigation/detection on risk can be
modeled easily.
- Provides a well-documented record of
improvements from corrective actions
implemented.
- Provides information useful in
developing test programs and in-line
monitoring criteria.
- Provides historical information useful
in analyzing potential product failures
during the manufacturing process.
- Provides new ideas for improvements
in similar designs or processes.
2 Risk Assessment
2.1 Define Goal of Risk Assessment
The first step in any risk assessment is to define the goal of the risk assessment.
Components of the FMEA are:
Severity: if a failure were to occur, what effect would that failure have on the
product quality and on the patient (if any)?
Probability of occurrence: how likely is it for a particular failure to occur?
Detectability: what mechanisms are in place (if any) to detect a failure if it were
to occur?
Each of the above metrics require clear definitions and a corresponding scale to rank
or score the projected impact (i.e. a scale for Severity; a scale for Probability; and a
scale for ability to Detect).. In addition, a composite score would then need to be
calculated (e.g. severity multiplied by Proabality multiplied by ability to detect ) and
matrixed so that final team recommendations are based on a calculated and
unambiguous fashion.
Page 2 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
2.2 Define overall process and process increments
(unit operations, steps)
Initiation of a FMEA requires the assembling of a
team usually comprised of a facilitator, a team lead,
and functional experts from development,
,manufacturing, quality, regulatory, etc., as
appropriate. The assembled team should first
describe the process in general unit operations, and
then section each unit operation into its component
parts. Use of a detailed flow chart of the process or
schematic of the design under evaluation is
recommended. It is also important that team
members gain input from key stakeholders who will
be responsible for implementing any resulting
recommendations to ensure feasibility.
FMEA Team Start-Up
1.Identify a facilitator, team lead and person
responsible for taking minutes and maintaining
records
2.Define the scope of the FMEA. Include a clear
definition of the product or process to be studied.
3.Assemble team members. Ensure that al keyl
impacted functional areas are represented, e.g.
development, manufacturing, quality, regulator,
etc.
4. Ensure that the necessary range of knowledge
and experience are represented on the teams( e.g.
is it necessary to have a skilled operator from
manufacturing involved instead of only engineers
or scientists?)
5.Define FMEA Teams boundaries of freedom What aspect of the FMEA is each team/ subteam
responsible for ?e.g. FMEA Analysis,
recommendations for improvement,
implementation of improvements, etc.?
6. Also define project deadline, team member
time constraints, etc. to ensure all are preparted
to support the initiative. 8.Do team members have
specific time constraints?
9. What is the procedure if team needs to expand
beyond the established boundaries?
Sectioning of the unit operation should be done in
process increments of sufficient size to enable
accurate risk assessment for each unit operation and
in turn the entire manufacturing train. It is critical to
describe each step in sufficient detail so that the
team can adequately assign the proper risk score to
the step. Careful attention to detail is needed so that
common practices that affect risk are captured in the
10.How should the FMEA be communicated
step description. While these additional levels of
to others?
detail provide greater accuracy, they also require
greater effort. The appropriate level of detail is a
matter of judgment for each team and depends on the perceived overall significance of the
project and the corresponding risk involved; for example introduction of a new technology
versus an established core competency.
2.3 Establish Clear Definitions
As indicated in Section 2.1, risk in an FMEA evaluation
has three components: Severity, Probability, and
Detectability. The definitions for the various levels of
severity, probability, and detectability should be clearly
articulated. Examples of definitions for each level are
presented in the tables below. For each of these risk
profile attributes, a five-point rating scale was
developed and utilized to ensure that the risk rating had:
appropriate levels of differentiation in order to
support meaningful risk prioritization
clearly apparent meaning in the context of the
overall risk to process and product quality
Page 3 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
FMEA
Is dependent on a complete sectioning of
the overall risk into risks associated with
the smallest increment step in the
process.
Requires an agreed-upon set of clearly
defined terms for the component parts of
risk (severity, probability, and
detectability), and the corresponding
scores.
Provides a means to determining the
level of attention to be applied to each
step, and summarizes recommendations
to reduce the risks. at each step
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
consistency across the risk profile attributes (severity, occurrence and detection) to
ensure that no attribute contributed disproportionately to the overall prioritization of
risk.
It is important to note that the definitions, levels and numbers assigned to the different levels
can vary depending on the system under evaluation or previously established definitions by
the organization or company. However, once a set of definitions are established for FMEA
evaluation of a system, the definitions should be piloted with a limited number of examples
to validate their clarity so as to build consistency across the unit operations within a project.
In general, the same rating scale should be applied to the components of severity, occurrence
and detection to avoid the appearance of skewing the resulting RPN (a calculation of the
assessed severity rating multiplied by occurrence rating and multiplied again by the detection
rating). Additionally, it has proven useful that nonconsecutive numbers (e.g. 1,3,5,7,10) are
more useful than consecutive numbers e.g, 1,2,3,4,5, as the use of non-consecutive numbers
allows more distinction between ratings and less debate amongst team members. As an
alternative, the team may want to put more emphasis on the severity criteria for example, in
which case a non-linear scoring scale can be utilized (e.g. 1, 4, 9,16, 25)
Note, the below definitions for Severity, Probability and Detection were developed for a FMEA
involving a new process development; these will change based on the subject under
assessment.
Severity Criteria for FMEA
In general, severity assesses how serious the effects would be should the potential risk occur. In
the example of a manufacturing process for a drug substance, the severity score is rated against
the impact of the effect caused by the failure mode on the batch quality. A non-linear scoring
scale can be applied to augment the effect of the severity criteria as shown in the table below.
Severity
Value
Description
Criteria
Irrelevant
No impact to product quality and process robustness
Slight
No impact to product quality
Important
Noticeable impact to product quality, but can be recovered by
reprocessing
16
Critical
Definite impact to product quality that may require rework
25
Disastrous
Batch failure, not recoverable by rework
Page 4 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
Probability of Occurrence Criteria for FMEA.
In general, the probability of occurrence evaluates the frequency that potential risk(s) will occur
for a given system or situation. The probability score is rated against the probability that the
effect occurs as a result of a failure mode. The example below applies a linear scoring scale to
the probability of occurrence of failure modes associated with the manufacturing process of a
drug substance.
Probability
Value
Description
An unlikely probability of occurrence
A remote probability of occurrence
An occasional probability of
occurrence
A moderate probability of occurrence
A high probability of occurrence
Page 5 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Criteria
Failure has never been seen in any relevant
lab experiments, or scale-up batches yet but
it is theoretically possible.
Failure only seen once or twice in relevant
lab experiments, never in scale-up batches.
Failure potential has been noted in several
relevant lab experiments, or at scale-up. If
procedures are followed the failure potential
is minimal.
Failure potential has been noted in several
relevant lab experiments, or at scale-up, inprocess control maybe required to avoid
failure.
Failure potential has been noted in several
relevant lab experiment, or at scale-up, an
active non-standard feed back control loop
may be required.
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
Detectability Criteria for FMEA.
In general, detectability is the probability of the failure being detected before the impact
of the failure to the system or process being evaluated is detected. The detectability score
is rated against the ability to detect the effect of the failure mode or the ability to detect
the failure mode it self. The example below applies a linear scoring scale to the
detectability criteria in an FMEA for a drug substance manufacturing process.
Detection
Value
Description
Criteria
High degree of
detectability
A: Validated automatic detection system that is a direct
measure of failure.
B: Two or more manual operated validated detection systems,
direct or indirect. (e.g. Control range and IPC)
Good
detectability
A: Single manually operated validated detection system that is
a direct measure of failure. (e.g. IPC of failure, validated PAT)
Likely to detect
A: Single manually operated validated detection system that is
not a direct measure of failure.
(e.g. PAT measurements or IPC's not directly linked to failure)
Fair detectability
A: Non validated (manual or automated) detection.
(e.g. visual level check, visual inspection of vessels).
Low or no
detectability
No ability to detect the failure
2.4 Risk score matrix
The composite risk score for each unit operation step is the product of its three
individual component ratings: severity, probability, and detectability. This composite
risk is called a risk priority number (RPN). .
RPN = S x P x D
The RPN number is not absolute and should be considered in context with other
factors that influence the product risk outside the scope of this evaluation. The RPN
provides a relative priority for taking action - the bigger the RPN, the more important
to address the corresponding failure being assessed.
Table 2.4 is an example of a FMEA Table/Form. For each formulation component or
manufacturing processing step under evaluation, the function of the component or
processing step, potential failure mode and effect of the failure mode should be
recorded. A severity score is then assigned. The root cause of the failure is described
and a score is assigned to the probability of occurrence of the failure. Controls that
are currently in place to detect the failure are listed and a detectability score is then
assigned. The RPN number is calculated. The action(s) that need to be taken to
reduce or mitigate the risk are listed and individuals or departments responsible for
implementing the actions are identified with target dates for completion.
Page 6 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
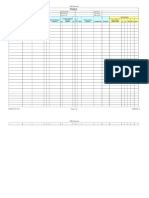
Table 2.4: EXAMPLE OF A FAILURE MODE AND EFFECTS ANALYSIS (FMEA) FORM
SYSTEM/PROJECT:
SUBSYSTEM/PROBLEM:
REFERENCE DRAWING:
CORE TEAM:
PREPARED BY:
Part/Proc
ess
Failure
Mode
Failure
Effects
(What is
the
function
of each
step)
(Describe
what could
go wrong)
(How
does the
failure
affect the
function
of the
step)
FMEA NUMBER:
FMEA DATE:
S
E
V
E
R
I
T
Y
Causes
O
C
C
U
R
R
E
N
E
(What is
the root
cause or
reason
for the
failure)
Controls
D
E
T
E
C
T
I
O
N
R
P
N
Actions
Responsibili
ty & Target
Completion
Date
Actions
taken
S
E
V
O
C
C
D
E
T
(What
controls
are
currently
in place
to catch
or
prevent
this
failure
3 Risk Control
There are two ways to control or reduce the risk at each unit operation step: mitigation and detection. In the
case of mitigation, the step is changed to reduce the impact of a failure (severity) or the likely hood
(probability) of a failure occurring. Detection refers to adding additional controls or feedback mechanisms
that can result in a reduction of either the severity or probability (or both).
3.1 Detection
Additional physical (e.g. inspection) and/or analytical tools can be added to a process that can
increase the ability to monitor the performance of the process, with or without real-time feedback.
Monitoring without feedback could reduce the probability of a failure, but real-time feedback will
reduce both the probability and severity of a failure. Implementation of such a feedback mechanism
is dependent on balance between the degree of risk reduction and cost/resource requirements.
Page 7 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
R
P
N
Training Guide: Failure Modes and Effects Analysis Guide
3.2 Mitigation options
A process step can be modified such that a failure become highly unlikely or that if there was a
failure, the impact to the patient is small. This typically requires identification of a root cause or
source of the failure, and removal of the root cause. This provides for a more robust process. By
identifying possible risk mitigation options and re-evaluating the results, teams can identify the
mitigation options that will have the most beneficial impact to overall risk.
4 Risk communication and Review
The outcome of the risk assessment and option analysis should be presented in an increasing level of detail.
This enables the reader of the report to focus on the recommendations and only delve into the details of
interest. This maintains the readers interest and ensures that relevant parts of report are given due scrutiny.
The outcome of a risk assessment and option analysis should be reviewed with all key stake holders to
ensure their understanding and buy-in, since they are usually responsible for subsequent implementation of
the recommendations. It is also important to stipulate any residual risks remaining after implementation.
Residual risks are risks that were identified as low as reasonably possible. These need periodic review to
ensure that the risks associated with affected unit operation steps do not creep from medium to high.
Page 8 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
5 Summary of Steps for Performing FMEA
1. Describe the product or process.
2. Review a block diagram of the product or flow chart of the process.
3. Complete the headers of FMEA table as customized for the specific need.
4. Break down the product or process into its components or steps and list each step or
component under the column with the header of Parts/Components in the FMEA
Table.
5. Identify all potential failure modes associated the product component or process
step.
6. List all potential failure modes for each item (product component or process step
under the Failure Mode column in the FMEA Table.
7. Describe the effects of each of the listed failure modes and assess the severity of
each of these effects on the product or process. Assign a severity rating to each
effect on the FMEA Table.
8. Identify the possible cause(s) of each failure mode.
9. Quantify the probability of occurrence of each of the causes of a failure mode.
10. Identify all existing controls (Current Controls) that contribute to the prevention of
the occurrence of each of the causes of a failure mode.
11. Determine the ability of each of the listed controls in preventing or detecting the
failure mode or its cause. Assign a ranking score to indicate the detection
effectiveness of each control.
12. Calculate the Risk Priority Number (RPN) = (SEV x OCC x DET).
13. Identify actions to address potential failure modes that have a high RPN
14. Assign an individual responsible for implementation of the defined action(s) and a
target date for completion.
15. After the defined actions have been implemented the overall effect on the failure
mode that the actions were supposed to address must be re-assessed and a new RPN
calculated.
16. The new RPN will help to determine if further action needs to be taken.
17. Update the FMEA Table every time there is a significant change in the product
design or process.
Page 9 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
Training Guide: Failure Modes and Effects Analysis Guide
References:
Mario Villacourt, Failure Mode and Effects Analysis (FMEA): A Guide for Continuous
Improvement for the Semiconductor Equipment Industry, technology Transfer #
92020963B-ENG, SEMATECH, 1992.
http://www.fmeainfocentre.com/handbooks/sematechsemiconductorfmeahandbook.pdf
Failure Modes and Effects Analysis (FMEA) Procedural Guide; Siliconfareast.com
http://www.siliconfareast.com/index.html
Design FMEA Scope; QualityTrainingPortal, Resource engineering, Inc
http:/www.qualitytrainingportal.com/resources/fmea/fmea_design_scope.htm
http:/www.qualitytrainingportal.com/resources/fmea/fmea_10step_dfmea.htm
http:/www.qualitytrainingportal.com/resources/fmea/fmea_process_scope.htm
http:/www.qualitytrainingportal.com/resources/fmea/fmea_10step_pfmea.htm
Page 10 of 10
DRAFT PROPOSAL NOT FOR IMPLEMENTATION
Date: May 29, 2008
Revision: 04
You might also like
- BS 887-2008 Precision Vernier Caliper Requirements and Test MethodsDocument20 pagesBS 887-2008 Precision Vernier Caliper Requirements and Test Methodszipaganredentorefrenii100% (1)
- 3L5W TemplateDocument2 pages3L5W TemplateOziel Cardenas100% (1)
- Kaizen Event Project Charter: Project Name Agency/Division/ Location Scope Meeting Date Target Event DateDocument3 pagesKaizen Event Project Charter: Project Name Agency/Division/ Location Scope Meeting Date Target Event DateDebashishDolonNo ratings yet
- Interface control document The Ultimate Step-By-Step GuideFrom EverandInterface control document The Ultimate Step-By-Step GuideNo ratings yet
- Kamishibai Intro PDFDocument27 pagesKamishibai Intro PDFGanesh Nagaraj100% (1)
- Expectations To Plastic Injection Molding SuppliersDocument59 pagesExpectations To Plastic Injection Molding SuppliersAbraham Reyna100% (4)
- Taurus Slim Series 9mmpt709Document19 pagesTaurus Slim Series 9mmpt709daks4u100% (1)
- 50 Ways To Improve Product Reliability - ARS Presentation 2011Document48 pages50 Ways To Improve Product Reliability - ARS Presentation 2011Vidyanand PattarNo ratings yet
- Failure Mode AND Effect Analysis: TPM Secretariat - Orai FactoryDocument27 pagesFailure Mode AND Effect Analysis: TPM Secretariat - Orai FactorySunilNo ratings yet
- 050 Diagnosis and Testing Vehicle Dynamic SuspensionDocument23 pages050 Diagnosis and Testing Vehicle Dynamic SuspensionsailorporNo ratings yet
- AIAG FMEA Manual The BenchmarkDocument2 pagesAIAG FMEA Manual The BenchmarkUmer Al-FaisalNo ratings yet
- FMEA Failure Modes Effects Analysis A Complete Guide - 2020 EditionFrom EverandFMEA Failure Modes Effects Analysis A Complete Guide - 2020 EditionNo ratings yet
- How To Conduct A Measurement Systems AnalysisDocument5 pagesHow To Conduct A Measurement Systems AnalysisNavnath TamhaneNo ratings yet
- Process FMEA (Failure Mode and Effect Analysis)Document17 pagesProcess FMEA (Failure Mode and Effect Analysis)hendra100% (1)
- White Paper: Introduction To Japanese Style Mizenboushi Methods For Preventing Problems Before They OccurDocument4 pagesWhite Paper: Introduction To Japanese Style Mizenboushi Methods For Preventing Problems Before They OccurEduardoNo ratings yet
- 203 LSS Gbo - FmeaDocument47 pages203 LSS Gbo - FmeaRocker byNo ratings yet
- Supplier PFMEA Workshop Rev 120814 OutvideoDocument32 pagesSupplier PFMEA Workshop Rev 120814 OutvideoVladimir Rodriguez Beltran100% (1)
- Notes of CQI 23 Ford Specific AssessmentDocument14 pagesNotes of CQI 23 Ford Specific AssessmentMuruganandhanNo ratings yet
- Agenda: Introduction To Reliability Asset Management System Reliability Equate Terms Failure Pareto PrincipleDocument26 pagesAgenda: Introduction To Reliability Asset Management System Reliability Equate Terms Failure Pareto PrincipleRengarajan ThiruvengadaswamyNo ratings yet
- Forever Requirements - ChryslerDocument35 pagesForever Requirements - ChryslerR H100% (1)
- Pillar: Hinshitsu Hozen or Quality MaintenanceDocument27 pagesPillar: Hinshitsu Hozen or Quality MaintenanceGREENEXE BUSINESS CONSULTANTNo ratings yet
- The FDA Group - The Guide To CAPA and Root Cause Analysis in FDA-Regulated IndustriesDocument34 pagesThe FDA Group - The Guide To CAPA and Root Cause Analysis in FDA-Regulated IndustriesAri Clecius100% (1)
- Failure Modes and Effects Analysis (FMEA) Quad Torc 23/05/2015Document3 pagesFailure Modes and Effects Analysis (FMEA) Quad Torc 23/05/2015Vishnu RoyNo ratings yet
- Diagnostic Troubleshooting Ebook - TSIADocument22 pagesDiagnostic Troubleshooting Ebook - TSIAAndrew CatesNo ratings yet
- Supplier Template Document For: - Process Flow Diagram, - Pfmea, - Control PlanDocument6 pagesSupplier Template Document For: - Process Flow Diagram, - Pfmea, - Control PlanPuneet SharmaNo ratings yet
- Shainin Vs Six SigmaDocument4 pagesShainin Vs Six Sigmabaro4518No ratings yet
- Cause Mapping Investigation Template - GeneralDocument20 pagesCause Mapping Investigation Template - GeneralMickloSoberanNo ratings yet
- Dodig m2 Quality D 2010 035Document54 pagesDodig m2 Quality D 2010 035MotherboardTVNo ratings yet
- Mistake Proofing ExamplesDocument33 pagesMistake Proofing ExamplesAhmad Bin Ismail KhanNo ratings yet
- RAM Guide 080305Document266 pagesRAM Guide 080305Ned H. CriscimagnaNo ratings yet
- Questions Being Addressed by This SpreadsheetDocument4 pagesQuestions Being Addressed by This SpreadsheetLuciano Marcelo OliveiraNo ratings yet
- FMEA TemplateDocument6 pagesFMEA TemplateHiếu Trần100% (1)
- A Case Study: A Process FMEA Tool To Enhance Quality and Efficiency of Manufacturing IndustryDocument8 pagesA Case Study: A Process FMEA Tool To Enhance Quality and Efficiency of Manufacturing IndustryBONFRINGNo ratings yet
- Process Potentail Failure Mode and Effects AnalysisDocument1 pageProcess Potentail Failure Mode and Effects Analysiscong daNo ratings yet
- APQPStatus Reporting GuidelinesDocument19 pagesAPQPStatus Reporting GuidelinesskluxNo ratings yet
- Fme (C) ADocument4 pagesFme (C) AMurat IslamNo ratings yet
- FMEA Memory JoggerDocument33 pagesFMEA Memory JoggeripatoffNo ratings yet
- A DMAIC Approach To Printed Circuit Board Quality Improvement-1 PDFDocument9 pagesA DMAIC Approach To Printed Circuit Board Quality Improvement-1 PDFrogers4759No ratings yet
- 7QC ToolsDocument48 pages7QC ToolsSudhagarNo ratings yet
- Fmea Methodology For Quality Improvement in Sheet Metal Industry IJERTV5IS010123Document5 pagesFmea Methodology For Quality Improvement in Sheet Metal Industry IJERTV5IS010123DanistergladwinNo ratings yet
- Critical Design ReviewDocument66 pagesCritical Design Reviewapi-314066443No ratings yet
- Fmea - Fmeca: Dr. Miha Mraz University of Ljubljana Faculty of Computer and Information Science LjubljanaDocument35 pagesFmea - Fmeca: Dr. Miha Mraz University of Ljubljana Faculty of Computer and Information Science LjubljanagkspNo ratings yet
- 8DDocument117 pages8DLokesh NarasimhaiahNo ratings yet
- Q M S (QMS) : Uality Anagement YstemDocument37 pagesQ M S (QMS) : Uality Anagement YstemDaud AliNo ratings yet
- Fracas Illustration1!2!130105114238 Phpapp01Document1 pageFracas Illustration1!2!130105114238 Phpapp01juancgr77No ratings yet
- Instructors GuideDocument108 pagesInstructors GuideLuiz IkedaNo ratings yet
- F543-07 Standard Specification and Test Methods For Metallic Medical Bone Screws1Document20 pagesF543-07 Standard Specification and Test Methods For Metallic Medical Bone Screws1Roberto Carlos Ramos Santillano100% (1)
- Six Sigma DMAIC MeasureDocument16 pagesSix Sigma DMAIC MeasureRaul Hoan DauNo ratings yet
- Pfmea Process Failure Mode and Effects AnalysisDocument34 pagesPfmea Process Failure Mode and Effects AnalysisRizky SihabNo ratings yet
- Evaluating FMEA, FMECA and FMEDADocument6 pagesEvaluating FMEA, FMECA and FMEDAZakaria RadaNo ratings yet
- Red Flags SurveyDocument6 pagesRed Flags Surveyhmp90No ratings yet
- FMEA failure modes effects analysis A Complete Guide - 2019 EditionFrom EverandFMEA failure modes effects analysis A Complete Guide - 2019 EditionNo ratings yet
- Interphex Manesty PAT TechnologyDocument33 pagesInterphex Manesty PAT TechnologyniknenadNo ratings yet
- Spraying Systems CatalogueDocument116 pagesSpraying Systems Catalogueniknenad50% (2)
- PC-11994 Aquarius Color ChartDocument2 pagesPC-11994 Aquarius Color ChartniknenadNo ratings yet
- Kollicoat Smartseal 30 D ShortDocument39 pagesKollicoat Smartseal 30 D ShortniknenadNo ratings yet
- PC-11642 Cavamax Native CyclodextrinsDocument4 pagesPC-11642 Cavamax Native CyclodextrinsniknenadNo ratings yet
- Kollicoat Smartseal 30 D ShortDocument39 pagesKollicoat Smartseal 30 D ShortniknenadNo ratings yet
- Monitoring Granulation Drying Using Near-Infrared SpectrosDocument6 pagesMonitoring Granulation Drying Using Near-Infrared SpectrosniknenadNo ratings yet
- PC-11792 Polyplasdone Ultra Ultra10Document2 pagesPC-11792 Polyplasdone Ultra Ultra10niknenadNo ratings yet
- 24 Laktoza MeggleDocument3 pages24 Laktoza MeggleniknenadNo ratings yet
- 250 42aECDocument35 pages250 42aECniknenadNo ratings yet
- PTR 037Document8 pagesPTR 037niknenadNo ratings yet
- Manesty Coating Presentation - 2009Document16 pagesManesty Coating Presentation - 2009niknenadNo ratings yet
- HTTP WWW - Dow.com PublishedLiterature DH 0081 0901b80380081505.pdf Filepath Polyox Pdfs Noreg 326-00013 PDFDocument12 pagesHTTP WWW - Dow.com PublishedLiterature DH 0081 0901b80380081505.pdf Filepath Polyox Pdfs Noreg 326-00013 PDFniknenadNo ratings yet
- FMC Coating EquipmentDocument26 pagesFMC Coating EquipmentniknenadNo ratings yet