Professional Documents
Culture Documents
Document 1
Uploaded by
Wee Choi ChiangCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Document 1
Uploaded by
Wee Choi ChiangCopyright:
Available Formats
13.
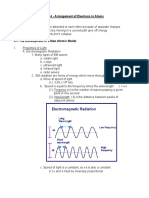
(a) These light contains respective gas in bulbs
1
- Atoms of gas get excited by electric discharge
- Electrons of the excited atoms returns to lower energy state and gives off energy 1
- There photons emit light according to the wavelength and types of gas in the 1
bulb
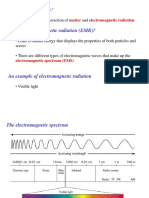
(b) Line emission spectrum
A series of bright lines of various colours/frequencies/wavelengths on a generally dark
background 1
Line absorption spectrum
A spectrum consisting of a series of dark lines superimposed on continuous spectrum 1
(c) (i) Consists of an electron revolving around (1) a positively charged nucleus/proton where the
nucleus is much heavier 1
e–
proton
(ii) Bohr’s postulate (Any two)
(1) Electron moves in certain allowed orbits (stable states) where the total
energy of the atom remains constant 1
(2) The angular momentum of the electon around the allowed orbit is quantized which in an
nh
interger multiple of 1
2
nh
Or L = nh or L=
2
You might also like
- 2019 - 2020 Chapter 2 Atomic Structure SK015Document71 pages2019 - 2020 Chapter 2 Atomic Structure SK015Jia En TanNo ratings yet
- 2.0 Atomic StructureDocument101 pages2.0 Atomic StructureTasya KassimNo ratings yet
- Atomic StructureDocument7 pagesAtomic StructureP. Phani prasadNo ratings yet
- Chapter 4 - Arrangement of Electrons in AtomsDocument7 pagesChapter 4 - Arrangement of Electrons in AtomsEmily Zuber 2023No ratings yet
- Chapter 2Document62 pagesChapter 2kere evaNo ratings yet
- ATOMIC STRUCTURE 1 - StudentDocument31 pagesATOMIC STRUCTURE 1 - Studentakepz.smaNo ratings yet
- CHME 222 - Lecture 6Document29 pagesCHME 222 - Lecture 6islam.lukmanov2003No ratings yet
- Chapter 1 Electronic Structure of AtomsDocument6 pagesChapter 1 Electronic Structure of AtomsHong Hong WongNo ratings yet
- Electronic Structure of Atoms (2023)Document66 pagesElectronic Structure of Atoms (2023)Tshegofatso DipaleNo ratings yet
- Chapter 2 Atomic StructureDocument88 pagesChapter 2 Atomic StructureHaifa amirahNo ratings yet
- Important Atomic Models: GeniusDocument37 pagesImportant Atomic Models: GeniusMoses AhmedNo ratings yet
- ATOMIC SPECTRA 2012 W AnswerDocument29 pagesATOMIC SPECTRA 2012 W AnswerAng chong bengNo ratings yet
- Bohr's Atomic Model.Document16 pagesBohr's Atomic Model.Vidhan SinghNo ratings yet
- 02 LASER and Fibre Optics FinalDocument33 pages02 LASER and Fibre Optics Finalnarayan patelNo ratings yet
- JEE Chemistry EssentialsDocument21 pagesJEE Chemistry EssentialsAdabala Durgarao NaiduNo ratings yet
- Type of SpectrumDocument5 pagesType of Spectrumfiza choudharyNo ratings yet
- AtomsDocument3 pagesAtomsCube PlayNo ratings yet
- Engineering Physics (PHY 1051)Document11 pagesEngineering Physics (PHY 1051)Harshita GauravNo ratings yet
- Components of Matter ExplainedDocument42 pagesComponents of Matter ExplainedEwartNo ratings yet
- Chapter 2 - Atomic - Structure - Part IDocument79 pagesChapter 2 - Atomic - Structure - Part I杨致远No ratings yet
- Chapter 2 Structure of AtomDocument2 pagesChapter 2 Structure of Atomgibinshaji2No ratings yet
- An Introduction To Molecular Orbital Theory Lectures 2018Document119 pagesAn Introduction To Molecular Orbital Theory Lectures 2018Nataliya LyutenkoNo ratings yet
- CHEM 1020: General Chemistry-I Lecture 3Document93 pagesCHEM 1020: General Chemistry-I Lecture 3Celeste SanchezNo ratings yet
- CHAPTER 2 Atomic Structure AND QUANTUM NUMBERDocument105 pagesCHAPTER 2 Atomic Structure AND QUANTUM NUMBERNurmaizatul AtikahNo ratings yet
- CH 08Document53 pagesCH 08SIKENo ratings yet
- Topic2 AtomicStructureDocument93 pagesTopic2 AtomicStructurenijamNo ratings yet
- Laser GoodDocument25 pagesLaser GoodOsaid AhmadNo ratings yet
- 2.1 Bohr's Student Version PDFDocument59 pages2.1 Bohr's Student Version PDFyeeterntanNo ratings yet
- Unit 1 Chemical Engineering-2Document72 pagesUnit 1 Chemical Engineering-2chirag malavNo ratings yet
- Atom ModelDocument26 pagesAtom ModelJoseph GuerreroNo ratings yet
- CHP 1 Basic Principle of ElectricityDocument22 pagesCHP 1 Basic Principle of ElectricityHasnain TanveerNo ratings yet
- 2.2 Electronic Configurations_HandoutDocument13 pages2.2 Electronic Configurations_HandoutTheodore KimNo ratings yet
- Electronic Structure of The AtomDocument55 pagesElectronic Structure of The AtomAlekhoy Pakz100% (1)
- 2.1 Bohr's Atomic ModelDocument81 pages2.1 Bohr's Atomic ModelAbdullah AhmadNo ratings yet
- Atomic StructureDocument27 pagesAtomic StructureNoor AhmedNo ratings yet
- AtomsDocument13 pagesAtomsRupa BalasubramanianNo ratings yet
- Atomic-structure-atomic-spectra-and-electron (1)Document11 pagesAtomic-structure-atomic-spectra-and-electron (1)techibu252No ratings yet
- Laser and Fiber Opticspdf - 240309 - 194703Document25 pagesLaser and Fiber Opticspdf - 240309 - 194703prachiahire75No ratings yet
- Junior ChemistryDocument72 pagesJunior ChemistryjahnavikidambiNo ratings yet
- Applied Chemistry (DAS 103) Unit - 1 Electron (E)Document9 pagesApplied Chemistry (DAS 103) Unit - 1 Electron (E)HRITIKNo ratings yet
- Chapter 4: Arrangement of Electrons in Atoms: Chapter 4.1: The Development of A New Atomic ModelDocument2 pagesChapter 4: Arrangement of Electrons in Atoms: Chapter 4.1: The Development of A New Atomic Modelrrr rrrNo ratings yet
- Topic 1Document30 pagesTopic 1Sanju Pradeep SantoshNo ratings yet
- CHM 131 Chapter 3Document90 pagesCHM 131 Chapter 3Syalin NorainNo ratings yet
- Stellar SpectraDocument37 pagesStellar SpectraSowgata ChowdhuryNo ratings yet
- General Chemistry Lecture 3Document80 pagesGeneral Chemistry Lecture 3Aaron Dela CruzNo ratings yet
- Che 1010 Lecture Notes - UpdatedDocument93 pagesChe 1010 Lecture Notes - Updatedtinashekeche816No ratings yet
- Atomic StructureDocument4 pagesAtomic StructureThea GermanNo ratings yet
- Lecture18 Optical PropertiesDocument43 pagesLecture18 Optical PropertiesPaula Andrea Nevado VelásquezNo ratings yet
- IB Chemistry - Atomic StructureDocument1 pageIB Chemistry - Atomic StructureNebula Is LiveNo ratings yet
- General Chemistry Lecture-Chapter 1Document78 pagesGeneral Chemistry Lecture-Chapter 1Bảo Long Trần LêNo ratings yet
- Thomson Atom Model: Physics NotesDocument13 pagesThomson Atom Model: Physics NotesEs ENo ratings yet
- Electron Configurations- Ionization EnergiesDocument33 pagesElectron Configurations- Ionization EnergiesRayan BotanyNo ratings yet
- Seminar Presentation of Radioactivity 3Document24 pagesSeminar Presentation of Radioactivity 3adelionqNo ratings yet
- NOTE: Bohr's ModelDocument43 pagesNOTE: Bohr's ModelmsccenterNo ratings yet
- Molecules and Condensed Matter: Modern Physics MA in Teaching College PhysicsDocument44 pagesMolecules and Condensed Matter: Modern Physics MA in Teaching College PhysicsLogan LeeNo ratings yet
- Physics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETDocument12 pagesPhysics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETsoyel afridiNo ratings yet
- NCERT PUNCH Chemistry Class 11 Complete Book Flattened SignedDocument304 pagesNCERT PUNCH Chemistry Class 11 Complete Book Flattened Signedsd0806787No ratings yet
- 26 Modern Physics Formula Sheets QuizrrDocument7 pages26 Modern Physics Formula Sheets QuizrrDhairya SharmaNo ratings yet
- UV - Vis Spectros PDFDocument25 pagesUV - Vis Spectros PDFNelson BarriosNo ratings yet
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenFrom EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillNo ratings yet
- TRIAL2012PAPER1 (Teachers) EditedDocument20 pagesTRIAL2012PAPER1 (Teachers) EditedWee Choi ChiangNo ratings yet
- April 2007 TestDocument12 pagesApril 2007 TestWee Choi ChiangNo ratings yet
- U6 March Test 2013Document7 pagesU6 March Test 2013Wee Choi ChiangNo ratings yet
- STPM Trial Physics 2Document18 pagesSTPM Trial Physics 2Wee Choi Chiang0% (1)
- Teknik of Answering QuestionDocument29 pagesTeknik of Answering QuestionWee Choi ChiangNo ratings yet
- NPC 2010 Winners ListDocument3 pagesNPC 2010 Winners ListWee Choi ChiangNo ratings yet
- THERMODYNAMICSDocument14 pagesTHERMODYNAMICSWee Choi ChiangNo ratings yet
- STPM Phys p1 2011Document14 pagesSTPM Phys p1 2011Acyl Chloride HaripremNo ratings yet
- Ipho 2011 - Workshop 1 - Google Map - Ukm Bangi Pangsapuri Ukm Taman TenagaDocument1 pageIpho 2011 - Workshop 1 - Google Map - Ukm Bangi Pangsapuri Ukm Taman TenagaWee Choi ChiangNo ratings yet
- Ipho Training Special Theory of RelativityDocument35 pagesIpho Training Special Theory of RelativityWee Choi ChiangNo ratings yet
- l6 End of Year Exam 2010 Paper 2 QuestionsDocument13 pagesl6 End of Year Exam 2010 Paper 2 QuestionschoichiangNo ratings yet
- Ipho 2011 - Workshop 1 - Google Map - Ukm Bangi Pangsapuri Ukm Taman TenagaDocument1 pageIpho 2011 - Workshop 1 - Google Map - Ukm Bangi Pangsapuri Ukm Taman TenagaWee Choi ChiangNo ratings yet