Professional Documents
Culture Documents
How to calculate the relative amounts of proeutectoid phase (α or Fe3C) and pearlite?
Uploaded by
Putra Teguh SitompulOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
How to calculate the relative amounts of proeutectoid phase (α or Fe3C) and pearlite?
Uploaded by
Putra Teguh SitompulCopyright:
Available Formats
FCC
BCC
BCT
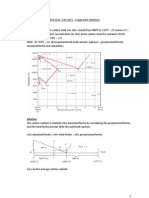
Fraction of pearlite: WP = X / (V+X) = (6.7 C1) / (6.7 0.76) Fraction of proeutectoid cementite: WFe3C = V / (V+X) = (C1 0.76) / (6.7 0.76)
Carbon pada Unit Cell FCC dan BCC ( BCT )
Calculate for hypoeutectoid alloy with composistion C0 !!!!!! Fraction of pearlite:
How to calculate the relative amounts of proeutectoid phase ( or Fe3C) and pearlite? (LEVER RULE) Example for hypereutectoid alloy with composition C1 Example Phases and Composition of Pearlite
% = 6.67 0.77 100 = 88.7% 6.67 0.0218 % Fe3C = 0.77 0.0218100 = 11.3% 6.67 0.0218
Fraction of proeutectoid Ferrite:
Calculate the amounts of ferrite and cementite present in pearlite. Since pearlite must contain 0.77% C, using the lever rule:
The effect of interlamellar spacing () of on the yield strength of pearlite
Phase Transformations. Kinetics.
Phase transformations (change of the microstructure) can be divided into three categories:
1.
Diffusion-dependent with no change in phase composition or number of phases present (e.g.melting, solidification of pure metal, allotropic transformations, recrystallization, etc.). Diffusion-dependent with changes in phase compositions and/or number of phases (e.g. eutectoid transformations). Diffusionless phase transformation - produces a metastable phase by cooperative small displacements of all atoms in structure (e.g. martensitic transformation discussed in later in this chapter).
2.
3.
Phase transformations do not occur instantaneously. Diffusiondependent phase transformations can be rather slow and the final structure often depend on the rate of cooling/heating. We need to consider the time dependence or kinetics of the phase transformations.
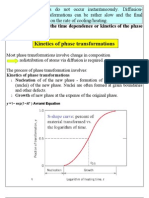
Kinetics of phase transformations
Most phase transformations involve change in composition redistribution of atoms via diffusion is required. The process of phase transformation involves: Kinetics of phase transformations 1. Nucleation of the new phase - formation of stable small particles (nuclei) of the new phase. Nuclei are often formed at grain boundaries and other defects. 2. Growth of new phase at the expense of the original phase.
y =1 exp(1ktn ) Avrami Equation
Let us consider eutectoid reaction as an example
eutectoid reaction:
(0.76 wt% C)
(0.022 wt% C) + Fe3C
The S-shaped curves are shifted to longer times at higher T showing that the transformation is dominated by nucleation. (nucleation rate increases with supercooling) and not by diffusion (which occurs faster at higher T)
Isothermal Transformation (or TTT) Diagrams (Temperature, Time, and % Transformation)
TTT
You might also like
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- TRANSIENT RESPONSE of AN RC CIRCUIT LAB REPORTDocument8 pagesTRANSIENT RESPONSE of AN RC CIRCUIT LAB REPORTJEJUNG92% (13)
- Chapt 09Document34 pagesChapt 09Jesse McClure75% (8)
- Fe-C Phase Diagram Lecture 33Document15 pagesFe-C Phase Diagram Lecture 33Manish GuptaNo ratings yet
- Chapter 10Document4 pagesChapter 10Izzat NasrullahNo ratings yet
- Phase Transformations: Ferrite BCCDocument61 pagesPhase Transformations: Ferrite BCCRajesh AutorajeshNo ratings yet
- Phase Diagram AssignmentDocument10 pagesPhase Diagram AssignmentSyafiqah RusdiNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument4 pagesIron-Iron Carbide Phase Diagram ExampleWanda Andreas WidyatmokoNo ratings yet
- Chapter 8 (Heat Treatment)Document12 pagesChapter 8 (Heat Treatment)Shekinah KanindaNo ratings yet
- Closed-Book Practice-Ch 10 (2017!08!08)Document12 pagesClosed-Book Practice-Ch 10 (2017!08!08)Juan0% (1)
- Material FinalDocument53 pagesMaterial FinalHuy Nguyễn Võ XuânNo ratings yet
- 4Document63 pages4ARJUN PESARU 19BME0161No ratings yet
- HW9 PracticexDocument7 pagesHW9 PracticexJod JDNo ratings yet
- Supporting water moleculesDocument20 pagesSupporting water moleculesHaibin SuNo ratings yet
- Mse230 E2 Practice SolDocument20 pagesMse230 E2 Practice SolAkshit PandeyNo ratings yet
- BakorDocument9 pagesBakorDilla WahabNo ratings yet
- MLE1101 - Tutorial 5 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 5 - Suggested SolutionsYin HauNo ratings yet
- Fe-C Phase Diagram MCQ & Essay QuestionsDocument5 pagesFe-C Phase Diagram MCQ & Essay QuestionsĐỗ ĐăngNo ratings yet
- Iron-Iron Carbide Phase Diagram ExampleDocument3 pagesIron-Iron Carbide Phase Diagram ExampleBenjamin Enmanuel Mango DNo ratings yet
- Chemical Reactors: DC DT RDocument8 pagesChemical Reactors: DC DT ROsas Jessica UwoghirenNo ratings yet
- Materials Science and Engineering Concept Check Part2 PDFDocument27 pagesMaterials Science and Engineering Concept Check Part2 PDF李宛芸No ratings yet
- Fe CDocument34 pagesFe CZaza ArifinNo ratings yet
- Week 11Document12 pagesWeek 11lduran_63No ratings yet
- Homework 14 Solutions Spring 2001Document2 pagesHomework 14 Solutions Spring 2001Ikhwan Wf Miscellaneous AveroesNo ratings yet
- Phase DiagramsDocument21 pagesPhase DiagramsabhishekNo ratings yet
- Phase TransformationsDocument27 pagesPhase TransformationsCatweazle999No ratings yet
- Introduction to Gray Cast Iron Brake Rotor MetallurgyDocument97 pagesIntroduction to Gray Cast Iron Brake Rotor MetallurgyBhargav Suvagiya100% (1)
- Chapter 10Document20 pagesChapter 10Dilip Singh ChoudharyNo ratings yet
- Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9Document34 pagesIron-Carbon Phase Diagram (A Review) See Callister Chapter 9Zefa Erliana YullahNo ratings yet
- Phase Diagram 3Document26 pagesPhase Diagram 3Tony StarkNo ratings yet
- Problems Phase FeDocument9 pagesProblems Phase FeAshutoshKumarNo ratings yet
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongNo ratings yet
- Assignment 8 SolutionDocument6 pagesAssignment 8 SolutionBrishen Hawkins100% (1)
- HW 5 - Due Thurs 110515 at 845am - Soln KeyDocument11 pagesHW 5 - Due Thurs 110515 at 845am - Soln Keyshayanebra100% (6)
- Time Temperature Transformation (TTT) Diagrams PDFDocument108 pagesTime Temperature Transformation (TTT) Diagrams PDFSerkan Apay100% (1)
- Practice Questions For Chapter 9 Material ScienceDocument5 pagesPractice Questions For Chapter 9 Material SciencedishfockendishNo ratings yet
- Assignment TwoDocument3 pagesAssignment TwoAnthony MubangaNo ratings yet
- CHEN3005 Process Instrumentation and ControlDocument4 pagesCHEN3005 Process Instrumentation and ControlVincent Ys TanNo ratings yet
- Isothermal reactor design for liquid phase reactionsDocument14 pagesIsothermal reactor design for liquid phase reactionsAnanda CahyaNo ratings yet
- Lection 5 (Eng) PDFDocument9 pagesLection 5 (Eng) PDFa320neoNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- ASSIGNMENT # 3 W KEYDocument16 pagesASSIGNMENT # 3 W KEYRashedul IslamNo ratings yet
- Phase Diagrams & Heat Treatment of Carbon SteelDocument84 pagesPhase Diagrams & Heat Treatment of Carbon SteelTanmay DuttaNo ratings yet
- Homework Ed Kinetics2Document5 pagesHomework Ed Kinetics2Edrian A. MañalongNo ratings yet
- ME2151 Tut1SolnDocument5 pagesME2151 Tut1SolnFlanc100% (1)
- PhysRevLett 100 183401Document4 pagesPhysRevLett 100 183401lfrnNo ratings yet
- Homework and Solutions - ch5 Ch6.IMSDocument18 pagesHomework and Solutions - ch5 Ch6.IMSHery RobiyantoroNo ratings yet
- CSTR FinalDocument36 pagesCSTR FinalMuhammad Yar KhanNo ratings yet
- Experiment 1B - Tubular ReactorDocument14 pagesExperiment 1B - Tubular ReactorNajmul Puda PappadamNo ratings yet
- Problem Set 6 SolutionDocument4 pagesProblem Set 6 SolutionRod De GuzmanNo ratings yet
- Material and Heat Treatment 3Document5 pagesMaterial and Heat Treatment 3lastking_king17No ratings yet
- EE 740 Professor Ali Keyhani Lecture #6: Load-Tap-Change Transformer (LTC)Document11 pagesEE 740 Professor Ali Keyhani Lecture #6: Load-Tap-Change Transformer (LTC)Mohamed A. HusseinNo ratings yet
- Ripple Current Reduction Using Multi-Dimensional Sliding Mode Control For Fuel Cell DC To DC Converter ApplicationsDocument6 pagesRipple Current Reduction Using Multi-Dimensional Sliding Mode Control For Fuel Cell DC To DC Converter ApplicationsJyothiPunemNo ratings yet
- Fundamentals Mathematical Modeling GuideDocument33 pagesFundamentals Mathematical Modeling GuidedeviNo ratings yet
- Pode PeteroDocument1 pagePode PeteroAgustin LuisNo ratings yet
- Chapter Outline: Phase Transformations. KineticsDocument23 pagesChapter Outline: Phase Transformations. Kineticsmpcd07No ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Biofilms in Bioelectrochemical Systems: From Laboratory Practice to Data InterpretationFrom EverandBiofilms in Bioelectrochemical Systems: From Laboratory Practice to Data InterpretationNo ratings yet
- Fundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionFrom EverandFundamentals of Electrocatalyst Materials and Interfacial Characterization: Energy Producing Devices and Environmental ProtectionNo ratings yet