Professional Documents
Culture Documents
2003 Trisomy9-Prenat Ultras Finding
Uploaded by
babydust34Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2003 Trisomy9-Prenat Ultras Finding
Uploaded by
babydust34Copyright:
Available Formats
Ultrasound Obstet Gynecol 2003; 22: 479483 Published online 29 August 2003 in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/uog.
233
Prenatal ultrasound ndings in complete trisomy 9
W. SEPULVEDA*, R. C. WIMALASUNDERA*, M. J. O. TAYLOR*, S. BLUNT, C. BE and S. DE LA FUENTE
*Centre for Fetal Care and Cytogenetics Laboratory, Queen Charlottes and Chelsea Hospital, Institute of Reproductive and Developmental Biology, Imperial College London, Hammersmith Campus, London, UK, Fetal Medicine Center, Department of Obstetrics and Gynecology and Cytogenetics Laboratory, Clinica Las Condes, Santiago and Clinica del Maule, Talca, Chile
K E Y W O R D S: fetal ultrasound; prenatal diagnosis; trisomy 9
ABSTRACT
Objective To report on the prenatal ultrasound ndings associated with complete trisomy 9. Methods Cases of complete trisomy 9 diagnosed prenatally were identied by reviewing the reports from two large cytogenetics laboratories serving tertiary referral centers for prenatal diagnosis. Information on prenatal ultrasound ndings and outcome was obtained in all cases. Results Nine cases of complete trisomy 9 were identied. The diagnosis was made in the rst trimester in four cases, in the second trimester in three and in the third trimester in two. Two fetuses underwent rst-trimester ultrasound screening for aneuploidy and the nuchal translucency thickness was increased in both. All ve fetuses detected in the second and third trimesters had several fetal anomalies including DandyWalker malformation in four cases, facial dysmorphism in four, genitourinary anomalies in three, congenital heart defects in three, ventriculomegaly in three, abnormal hands in two and megacisterna magna in one. Four fetuses were growth-restricted at the time of ultrasound evaluation. However, the two cases diagnosed in the third trimester had routine secondtrimester anomaly scans reported as normal. There were no survivors in this series. Conclusion Fetuses with complete trisomy 9 have multiple anomalies that can be readily detected prenatally by ultrasound. These mainly include, but are not restricted to, craniofacial, cardiovascular, musculoskeletal and genitourinary malformations. However, ndings can be subtle and therefore missed at the routine second-trimester scan. Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
INTRODUCTION
Trisomy 9 is an uncommon chromosomal disorder that was rst described by Feingold and Atkins in 19731 . It is characterized by the presence of multiple structural anomalies involving primarily the brain, face, heart, kidneys and limbs in association with neurodevelopmental delay and growth deciency1 3 . From the cytogenetic point of view, cases of trisomy 9 present either in complete (non-mosaic) or mosaic states3 5 , although they may also be the result of de novo mutation or familial balanced rearrangement in less common cases of partial trisomy 9 syndromes6 . The vast majority of trisomy 9 pregnancies end in early miscarriage and are diagnosed only if routine karyotyping of products of conception is performed7 . Rarely, trisomy 9 progresses into later stages of pregnancy, when characteristic features can be detected by prenatal ultrasound8 . However, the fact that most cases reaching the second and third trimester represent mosaic rather than non-mosaic forms3 5 makes the prenatal ultrasound diagnosis of complete trisomy 9 exceptionally rare. Several authors have reported on the prenatal ultrasound ndings in complete trisomy 9, for a total of eight cases reported to date8 14 . However, these reports were based on anecdotal observations from isolated cases, thus limiting the information available for prenatal screening and counseling. Furthermore, in most prenatally diagnosed cases of trisomy 9 reported in the literature the diagnosis was made incidentally by fetal karyotyping for routine indications such as maternal age, followed by termination of pregnancy (TOP)15,16 , and only limited information on the actual prenatal ultrasound ndings were available. In this report we review nine cases of complete trisomy 9, with special emphasis on ultrasound features leading to the prenatal diagnosis of this condition.
Correspondence to: Prof. W. Sepulveda, Centre for Fetal Care, Queen Charlottes and Chelsea Hospital, Hammersmith Hospitals NHS Trust, Du Cane Road, London W12 0HS, UK (e-mail: waldosep@hotmail.com) Accepted: 26 March 2003
Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
ORIGINAL PAPER
480
Sepulveda et al. cases was then obtained by reviewing the corresponding medical records and ultrasound reports. Fetal samples for karyotyping were collected by standard techniques including chorionic villus sampling (CVS), amniocentesis or fetal blood sampling under continuous ultrasound guidance. Cases diagnosed in the rst trimester were counseled regarding the possibility of mosaicism, conned placental mosaicism or pseudomosaicism, and further fetal analysis by amniocentesis and/or fetal blood sampling was offered15 . A second-trimester
METHODS
The reports from two large cytogenetics laboratories serving tertiary referral centers for prenatal diagnosis in London, UK and Santiago, Chile were reviewed for cases of complete trisomy 9 diagnosed prenatally. For the purposes of this study, complete trisomy 9 was diagnosed when two independent culture asks demonstrated trisomy 9 in all fetal cells analyzed17 . The relevant clinical and ultrasound information from these
Table 1 Prenatal ultrasound ndings in complete trisomy 9 Maternal Gestational age age Case (years) (weeks) 1 42 11 14 16
Reason for referral
Ultrasound ndings
Procedure CVS AC
Karyotype (cells counted)
Outcome
38
12 16
3 4
41 40
12 12 24
31
19
33
20
28
21
23
26
32
36
33
Previous trisomy 13 None noted Repeat scan Nuchal edema Repeat scan IUGR, ventriculomegaly, bilateral pleural effusions, mild pericardial effusion, mild ascites, bilateral hydronephrosis Increased NT NT thickness 4.5 mm Repeat scan Ventriculomegaly, absent cerebellar vermis, nuchal edema, hypoplastic left heart, bilateral polydactyly, rocker-bottom feet, short femora Advanced MA None noted Advanced MA NT thickness 3 mm, reverse ow in ductus venosus Repeat scan IUGR, brachycephaly, diaphragmatic hernia, mediastinal shift, bilateral hydronephrosis Fetal anomalies Severe oligohydramnios, dolicocephaly, absent cerebellar vermis, echogenic bowel, dysplastic kidneys, unilateral hydronephrosis, small bladder, absent EDF-UA Fetal anomalies IUGR, strawberry-shaped head, ventriculomegaly, absent cerebellar vermis, dysmorphic face, micrognathia, nuchal edema, mild generalized subcutaneous edema, bilateral hydronephrosis, echogenic kidneys, echogenic bowel, overlapping ngers Fetal anomalies IUGR, anhydramnios, dysplastic polycystic horsehoe kidney, absent bladder, atrial septal defect Repeat scan Dysmorphic face, bilateral microphthalmia, absent cerebellar vermis, umbilical vein varix Small for dates IUGR, oligohydramnios, ventriculomegaly, absent cerebellar vermis, hypotelorism, hypoplastic left heart, narrow chest, reduced EDF-UA Fetal anomalies IUGR, oligohydramnios, ventriculomegaly, megacisterna magna, microphthalmia, hypotelorism, hypoplastic left heart, overlapping ngers, single umbilical artery
47,XX,+9 (40) TOP 16 weeks 47,XX,+9 (118)
CVS
47,XY,+9 (30)
TOP 17 weeks
CVS CVS
47,XY,+9 (32) 47,XX,+9 (22)
IUD 17 weeks IUD 31 weeks
AC
47,XY,+9 (20)
TOP 20 weeks
FBS
47,XX,+9 (30)
IUD 33 weeks
47,XY,+9 (50)* IUD 36 weeks
FBS
FBS
47,XY,+9 (36)
Delivery 36 weeks, NND day 4
AC, amniocentesis; CVS, chorionic villus sampling; EDF-UA, end-diastolic ow in umbilical artery; FBS, fetal blood sampling; IUD, intrauterine death; IUGR, intrauterine growth restriction; MA, maternal age; NND, neonatal death; NT, nuchal translucency; TOP, termination of pregnancy. *47,XY,+der(9)(q32qter)mat karyotype according to postnatal samples.
Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
Ultrasound Obstet Gynecol 2003; 22: 479483.
Prenatal ultrasound in trisomy 9 ultrasound scan was also offered in order to determine the type of malformations present in the fetus. In such cases, prenatal ultrasound ndings were reported as those obtained before and after the karyotype results became available. Cases primarily detected in the second and third trimesters were managed according to the type and severity of malformations present in the fetus, the parents being fully informed about the prognostic implications of the prenatal ndings. In Chile, because the current law does not allow elective TOP, cases were managed expectantly with serial ultrasound scans for viability and fetal growth. In order to conrm the prenatal diagnosis, postnatal samples from placenta, skin or cardiac puncture were obtained where possible.
481
RESULTS
During a 12-year period from 1990 to 2002, nine cases of complete trisomy 9 were identied, ve from Queen Charlottes and Chelsea Hospital in London and four from Clinica Las Condes in Santiago. One of these cases was previously reported12 . In all cases the culture reported only trisomy 9 cell lines after analysis of a median of 30 (range, 1550) cells. Information on the relevant clinical and ultrasound ndings is presented in Table 1. The diagnosis was made in the rst trimester in four cases, in the second trimester in three and in the third trimester in two. Procedures used for the primary diagnosis included CVS in ve cases, fetal blood sampling in three and amniocentesis in one. Among the four cases diagnosed in the rst trimester, two had ultrasound screening based on maternal age and nuchal translucency (NT) measurement18 revealing increased risk of trisomy 21 of 1 : 12 and 1 : 3, respectively. The other two cases evaluated in the rst trimester underwent elective karyotyping because of previous trisomy 13 in one and advanced maternal age in the other, with no information on the actual NT measurement being available. Follow-up second-trimester ultrasound scans in these cases conrmed multiple congenital anomalies in three cases and intrauterine demise in another. Prenatal ultrasound was the primary diagnostic method used in the remaining ve cases, which all showed gross fetal abnormalities including DandyWalker malformation in four cases, genitourinary anomalies in three, ventriculomegaly in three and congenital heart defects in three (Figure 1). Additional dysmorphic features of the fetal face and hand were seen in four and two cases, respectively, and megacisterna magna was noted in one. Four fetuses were growth-restricted at the time of ultrasound evaluation. However, the two cases diagnosed in the third trimester had routine second-trimester anomaly scans reported as normal, but subsequent ultrasound assessment performed for a clinical suspicion of fetal growth restriction revealed multiple congenital anomalies in both. Importantly, two cases had second-trimester maternal serum screening, which yielded a low-risk result for aneuploidy in both.
Complete pregnancy follow-up was available in all cases. Three women opted for elective TOP according to the UK Abortion Act. There were no survivors from the six continuing pregnancies, with ve intrauterine deaths, either in the second (n = 2) or third trimester (n = 3), and one early neonatal death. The diagnosis of complete trisomy 9 was conrmed by amniocentesis in one case and by cytogenetic study from the placenta or postmortem skin biopsy in three. In an additional case postmortem samples from fetal skin and intracardiac blood showed a 47,XY,+der(9) karyotype in all cells analyzed, with a deletion at q32qter, this region being replaced by chromosomal material of unknown origin. Subsequent parental karyotyping conrmed maternal balanced reciprocal translocation involving chromosomes 9 and 10, resulting in a 46,XX,t(9;10)(q32;p13) karyotype. Results from the remaining cases were not obtained due to degree of maceration of fetal skin after prolonged intrauterine death and culture failure. Full postmortem examination was available in three cases and, with the exception of central nervous system anomalies, all prenatal ndings were conrmed.
DISCUSSION
We report the largest series describing the prenatal ultrasound ndings in fetuses with complete trisomy 9. This was possible by collecting cases from two large cytogenetics laboratories during a 12-year period, although based in different clinical settings. In Chile there is no formal screening for aneuploidy during pregnancy, as elective TOP is not allowed under any circumstances. In spite of this limitation, information provided by these cases is important as the natural history of complete trisomy 9 can be accurately delineated. In contrast, in the UK there are several screening programs for aneuploidy and TOP is an option. This was reected in the diagnostic and management approach in these cases, with the main indications for karyotyping being abnormal ndings at ultrasound scan in four cases and previous history of aneuploidy in the other. Accordingly, three of the ve women opted for elective TOP once the diagnosis was conrmed. Nevertheless, despite antenatal screening, the two cases that had second-trimester serum screening were both reported as low risk. Information on maternal serum analytes in second-trimester pregnancies with complete trisomy 9 is scarce, but a dramatic decrease in human chorionic gonadotropin (hCG) levels has been reported10,19 . In our cases hCG levels were only mildly decreased in one but normal in the other. In addition, two women had second-trimester ultrasound screening that reported no obvious fetal anomalies. There is no clear explanation for missing the diagnosis in the second trimester in these cases. However, one of the women underwent scanning in 1990, when ultrasound resolution could be such that the ability to detect structural anomalies may have been limited. The other case had a nondetailed second-trimester scan performed by her private obstetrician.
Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
Ultrasound Obstet Gynecol 2003; 22: 479483.
482
Sepulveda et al.
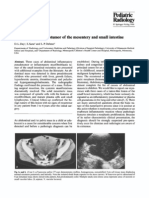
Figure 1 Prenatal ultrasound ndings at 16 weeks gestation in a fetus with trisomy 9. (a) DandyWalker malformation. (b) Abnormal prole with bulbous nose and micrognathia. (c) Abnormal four-chamber view of the heart and intracardiac echogenic focus. (d) Polydactyly.
Trisomy 9 is characterized by a constellation of ndings including mainly craniofacial, cardiovascular, musculoskeletal, genitourinary and central nervous system malformations1 5 . In most of the reported cases, however, information on the phenotype of this condition was obtained postnatally. As shown in this report, complete trisomy 9 is associated with a high intrauterine lethality rate, so the information obtained by pediatric geneticists may have undergone signicant bias as survivors are most probably those affected with the less serious malformations. From the prenatal point of view, the main phenotypic characteristics of trisomy 9 overlap those of trisomy 188 , with the most striking ultrasound ndings being defects of the cerebellar vermis, genitourinary anomalies and congenital heart defects, usually in association with intrauterine growth restriction (IUGR)8 14 . Dysmorphic features in the fetal face and limbs, although producing characteristic postnatal ndings1 5 , were seen prenatally by ultrasound in only a few of our cases. Associated reduction or absence of amniotic uid could
explain, at least in part, the low detection of these features antenatally. Most of our cases had the diagnosis of trisomy 9 conrmed further on amniotic uid or postnatal samples. However, pseudomosaicism or conned placental mosaicism is well recognized with trisomy 915,20 . These conditions are more likely to be found when the original cytogenetic sample is chorionic villi. Although there are few data on rst-trimester NT detection of trisomy 911,13 , the two women in our cohort who underwent NT screening were both detected as high risk. With the increasing popularity of NT screening for fetal aneuploidy18 , it seems reasonable to assume that the majority of trisomy 9 fetuses would be detected in the rst trimester. Therefore, trisomy 9 would increasingly be initially diagnosed by CVS. Previous groups have suggested that in every case of trisomy 9 diagnosed on chorionic villi the parents should be counseled regarding the risk of pseudomosaicism or conned placental mosaicism, and offered further fetal analysis by amniocentesis and/or fetal blood sampling and detailed
Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
Ultrasound Obstet Gynecol 2003; 22: 479483.
Prenatal ultrasound in trisomy 9 ultrasound assessment15 . The uncultured amniocytes or blood lymphocytes can be rapidly analyzed using uorescent in situ hybridization for chromosome 9 as well as standard culture20 . All cases of complete trisomy 9 diagnosed on amniocytes or lymphocytes have been associated with ultrasound anomalies15 and, where relevant, the option of TOP should be offered to the parents. Counseling for mosaic trisomy 9 is far more difcult. The presence of mosaic trisomy 9 at fetal blood sampling, even when the rate of mosaicism is low, must be considered abnormal15 . In cases of mosaic trisomy 9 on amniocytes, further fetal analysis by fetal blood sampling should be offered15 . Alternatively, in contrast to rst-trimester cytogenetic diagnosis of trisomy 9, a more detailed examination of the fetal anatomy by ultrasound is possible in the second trimester. This would help in conrming the cytogenetic diagnosis and, therefore, detailed fetal ultrasound should be mandatory. Similarly, if amniocentesis following a CVS diagnosis of trisomy 9 shows a normal karyotype, further ultrasound surveillance for IUGR and congenital anomalies should be offered. If TOP is performed fetal blood and skin should be sent to conrm the diagnosis of mosaic or non-mosaic trisomy 9. Likewise if a newborn infant has phenotypic features of trisomy 9, karyotyping must be performed on both lymphocytes and broblasts because of the degree of variability of mosaicism usually present in these cases. In conclusion, in our experience the cytogenetic diagnosis of trisomy 9 associated with fetal anomalies were always conrmed as complete trisomy 9. Therefore, ultrasound plays an important role in the counseling of these pregnancies as abnormal ultrasound ndings should help in the conrmation of the cytogenetic diagnosis. Conversely, normal ultrasound ndings in the presence of a diagnosis of trisomy 9 should prompt further fetal surveillance and karyotyping.
483
anomalies in the rst diagnosed case. Hum Pathol 1974; 5: 223232. Jones KL. Trisomy 9 mosaic syndrome. In Smiths Recognizable Patterns of Human Malformation (5th edn). Saunders: Philadelphia, PA, 1997; 2829. Arnold GL, Kirby RS, Stern TP, Sawyer JR. Trisomy 9: review and report of two new cases. Am J Med Genet 1995; 56: 252257. Cantu ES, Eicher DJ, Pai GS, Donahue CJ, Harley RA. Mosaic vs. nonmosaic trisomy 9: report in a liveborn infant evaluated by uorescence in situ hybridization and review of the literature. Am J Med Genet 1996; 62: 330335. Smart RD, Viljoen DL, Fraser B. Partial trisomy 9 further delineation of the phenotype. Am J Med Genet 1988; 31: 947951. Boue J, Boue A, Deluchat C. Identication of C trisomies in human abortuses. J Med Genet 1975; 12: 265268. Benacerraf BR, Pauker S, Quade BJ, Bieber FR. Prenatal sonography in trisomy 9. Prenat Diagn 1992; 12: 175181. McDufe RS. Complete trisomy 9: case report with ultrasound ndings. Am J Perinatol 1994; 11: 8084. Roshanfekr D, Dahl-Lyons C, Pressman E, Ural S, Blakemore K. Complete trisomy 9 in a term fetus: a case report. J Matern Fetal Med 1998; 7: 247249. Pinette MG, Pan Y, Chard R, Pinette SG, Blackstone J. Prenatal diagnosis of nonmosaic trisomy 9 and related ultrasound ndings at 11.7 weeks. J Matern Fetal Med 1988; 7: 4850. Sandoval R, Sepulveda W, Gutierrez J, Be C, Altieri E. Prenatal diagnosis of nonmosaic trisomy 9 in a fetus with severe renal disease. Gynecol Obstet Invest 1999; 48: 6972. Murta C, Moron A, Avila M, Franca L, Vargas P. Reverse ow in the umbilical vein in a case of trisomy 9. Ultrasound Obstet Gynecol 2000; 16: 575577. Yeo L, Waldron R, Lashley S, Day-Salvatore D, Vintzileos AM. Prenatal sonographic ndings associated with nonmosaic trisomy 9 and literature review. J Ultrasound Med 2003; 22: 425430. Saura R, Traore W, Taine L, Wen ZQ, Roux D, MaugeyLaulom B, Rufe M, Vergnaud A, Horovitz J. Prenatal diagnosis of trisomy 9. Six cases and a review of the literature. Prenat Diagn 1995; 15: 609614. Chitayat D, Hodgkinson K, Luke A, Winsor E, Rose T, Kalousek D. Prenatal diagnosis and fetopathological ndings in ve fetuses with trisomy 9. Am J Med Genet 1995; 56: 247251. Hsu LYF, Perlis TE. United States survey on chromosome mosaicism and pseudomosaicism in prenatal diagnosis. Prenat Diagn 1984; 4: 97130. Nicolaides KH, Sebire NJ, Snijders RJM. The 1114 Week Scan. The Diagnosis of Fetal Abnormalities. Parthenon Publishing: Carnforth, UK, 1999. Lam YH, Lee CP, Tang MHY. Low second-trimester maternal serum human chorionic gonadotrophin in a trisomy 9 pregnancy (Letter). Prenat Diagn 1998; 18: 1212. Van den Berg C, Ramlakhan SK, Van Opstal D, Brandenburg H, Halley DJ, Los FJ. Prenatal diagnosis of trisomy 9: cytogenetic, FISH, and DNA studies. Prenat Diagn 1997; 17: 933940.
3.
4.
5.
6.
7. 8. 9. 10.
11.
12.
13.
14.
15.
16.
17.
ACKNOWLEDGMENT
This work was supported by Sociedad Profesional de Medicina Fetal Fetalmed Limitada, Chile.
18.
19.
REFERENCES
1. Feingold M, Atkins L. Case report: a case of trisomy 9. J Med Genet 1973; 10: 184187. 2. Kurnick J, Atkins L, Feingold M, Hills J, Dvorak A. Trisomy 9: predominance of cardiovascular, liver, brain, and skeletal 20.
Copyright 2003 ISUOG. Published by John Wiley & Sons, Ltd.
Ultrasound Obstet Gynecol 2003; 22: 479483.
You might also like
- First-Trimester Ultrasound Nuchal Translucency Screening For Fetal AneuploidyDocument37 pagesFirst-Trimester Ultrasound Nuchal Translucency Screening For Fetal AneuploidyCTAFDocumentsNo ratings yet
- Nicolaides The 11-13 Weeks Scan 2004Document113 pagesNicolaides The 11-13 Weeks Scan 2004ScopulovicNo ratings yet
- Thermal Destruction of Microorganisms in 38 CharactersDocument6 pagesThermal Destruction of Microorganisms in 38 CharactersRobin TanNo ratings yet
- 2004, Vol.31, Issues 1, Ultrasound in ObstetricsDocument213 pages2004, Vol.31, Issues 1, Ultrasound in ObstetricsFebrinata MahadikaNo ratings yet
- Anatomy Yoga Therapy Conf ManualDocument84 pagesAnatomy Yoga Therapy Conf ManualPablo ErpelNo ratings yet
- Ultrasound Scanning of Fetal AnomalyDocument19 pagesUltrasound Scanning of Fetal AnomalyFA Khan0% (1)
- Ultrasound Obstetric and Gynecologic PDFDocument190 pagesUltrasound Obstetric and Gynecologic PDFAndrei MurariuNo ratings yet
- Second MbbsDocument2 pagesSecond MbbsPriya SelvarajNo ratings yet
- Prune Belly SyndromeDocument39 pagesPrune Belly SyndromeHudaNo ratings yet
- An Atlas of Neonatal Brain Sonography, 2nd EditionFrom EverandAn Atlas of Neonatal Brain Sonography, 2nd EditionRating: 5 out of 5 stars5/5 (5)
- Cell Organelle Review Worksheet 14-15Document2 pagesCell Organelle Review Worksheet 14-15Kirsten Troupe100% (1)
- Environmental Science MIDTERMDocument4 pagesEnvironmental Science MIDTERMGomez Agustin LeslieNo ratings yet
- Ovarian TorsiDocument8 pagesOvarian TorsiSebastian GandyNo ratings yet
- Molar Pregnancy PDFDocument8 pagesMolar Pregnancy PDFEgaSuharnoNo ratings yet
- 10 1002@uog 21956Document8 pages10 1002@uog 21956Felix CamposNo ratings yet
- Prenatal Management, Pregnancy and Pediatric Outcomes in Fetuses With Septated Cystic HygromaDocument5 pagesPrenatal Management, Pregnancy and Pediatric Outcomes in Fetuses With Septated Cystic HygromawitaNo ratings yet
- Kelainan JaninDocument6 pagesKelainan JaninIntan PermataNo ratings yet
- AdamDocument29 pagesAdamPrasetio Kristianto BudionoNo ratings yet
- Cog NTDocument11 pagesCog NTFabiolaQuijaiteNo ratings yet
- Gim 20047Document4 pagesGim 20047Sabhina AnseliaNo ratings yet
- Detecting fetal abnormalities at 11-14 weeksDocument2 pagesDetecting fetal abnormalities at 11-14 weeksKeith Mark AlmarinesNo ratings yet
- Referensi No 7Document5 pagesReferensi No 7Bagus Wanda HabibullahNo ratings yet
- Management of Monochorionic Twin Pregnancy: Green-Top Guideline No. 51Document13 pagesManagement of Monochorionic Twin Pregnancy: Green-Top Guideline No. 51indra_strongNo ratings yet
- Sacrococcygeal TeratomaDocument21 pagesSacrococcygeal TeratomaEm VelascoNo ratings yet
- 6 - Preterm Pregnancy With Sacrococcygeal TeratomaDocument5 pages6 - Preterm Pregnancy With Sacrococcygeal TeratomarobertuserikkantonaNo ratings yet
- Papers: Eclampsia The United KingdomDocument6 pagesPapers: Eclampsia The United KingdomsmileyginaaNo ratings yet
- AAOGUDocument6 pagesAAOGUGoran JosipovićNo ratings yet
- Advancesinsurgeryfor Abdominalwalldefects: Gastroschisis and OmphaloceleDocument12 pagesAdvancesinsurgeryfor Abdominalwalldefects: Gastroschisis and OmphaloceleSitti HazrinaNo ratings yet
- Choledochal CystDocument5 pagesCholedochal CystZulia Ahmad BurhaniNo ratings yet
- Amnioinfusion Compared With No Intervention In.18Document8 pagesAmnioinfusion Compared With No Intervention In.18owadokunquick3154No ratings yet
- Diagnosis of Polycystic Ovarian 9 Syndrome in AdolescenceDocument17 pagesDiagnosis of Polycystic Ovarian 9 Syndrome in AdolescenceTriratna FauziahNo ratings yet
- Fetal Structural Anomaly Screening at 11-14 Weeks of Gestation at Maharaj Nakorn Chiang Mai HospitalDocument6 pagesFetal Structural Anomaly Screening at 11-14 Weeks of Gestation at Maharaj Nakorn Chiang Mai HospitalobgynlaosNo ratings yet
- Materials and MethodsDocument1 pageMaterials and MethodsImtihanamiseNo ratings yet
- P15:Secondandthirdtrimestersi: S. Latella, V. Fenu, P. Ceccarelli, F. Baldinotti, L. Iughetti, A. Volpe, V. MazzaDocument2 pagesP15:Secondandthirdtrimestersi: S. Latella, V. Fenu, P. Ceccarelli, F. Baldinotti, L. Iughetti, A. Volpe, V. MazzaXlr8zzNo ratings yet
- Ultrasound Study of Ovarian Cysts in Pregnancy Prevalence and SignificanceDocument4 pagesUltrasound Study of Ovarian Cysts in Pregnancy Prevalence and SignificanceDavid Eka PrasetyaNo ratings yet
- PMDS, Polysplenia, and Short Pancreas CaseDocument13 pagesPMDS, Polysplenia, and Short Pancreas CaseDinesh ThapaNo ratings yet
- Hamil AnggurDocument7 pagesHamil AnggurIchunx MelissaNo ratings yet
- SimonyiDocument11 pagesSimonyiPrasetio Kristianto BudionoNo ratings yet
- 10.1.1.546.6206 BedahDocument7 pages10.1.1.546.6206 Bedahfikri hanifNo ratings yet
- Clinical Presentation of Childhood Leukaemia: A Systematic Review and Meta-AnalysisDocument9 pagesClinical Presentation of Childhood Leukaemia: A Systematic Review and Meta-AnalysisPrima YosiNo ratings yet
- Full TextDocument1 pageFull TexthimNo ratings yet
- Circ PlacentDocument7 pagesCirc PlacentnicoavilaNo ratings yet
- Rupture of Tubal Pregnancy in The Vilnius Population: Pasquale Berlingieri, Grazina Bogdanskiene, Jurgis G. GrudzinskasDocument4 pagesRupture of Tubal Pregnancy in The Vilnius Population: Pasquale Berlingieri, Grazina Bogdanskiene, Jurgis G. Grudzinskaslilis lestariNo ratings yet
- Aydin 2017Document7 pagesAydin 2017Berry BancinNo ratings yet
- Mansfield Et Al-1999-Prenatal DiagnosisDocument6 pagesMansfield Et Al-1999-Prenatal DiagnosisM T Lê VănNo ratings yet
- Findings in Uterine Biopsies Obtained by Laparotomy From Bitches With Unexplained Infertility or Pregnancy Loss: An Observational StudyDocument11 pagesFindings in Uterine Biopsies Obtained by Laparotomy From Bitches With Unexplained Infertility or Pregnancy Loss: An Observational Studyrafael molina bernalNo ratings yet
- Kista OvariumDocument4 pagesKista OvariumAde Gustina SiahaanNo ratings yet
- Spinal Tuberculosis in Children: Sarah Eisen, Laura Honywood, Delane Shingadia, Vas NovelliDocument7 pagesSpinal Tuberculosis in Children: Sarah Eisen, Laura Honywood, Delane Shingadia, Vas NovelliAnonymous YaqenULNgNo ratings yet
- JMedLife 09 297 4Document5 pagesJMedLife 09 297 4verotugasNo ratings yet
- Ultrasound diagnostic study acute salpingitisDocument11 pagesUltrasound diagnostic study acute salpingitisAhmad Arbi AninditoNo ratings yet
- Changed Indications For Cesarean Sections: Ylva Vladic Stjernholm, Karin Petersson & Eva EnerothDocument5 pagesChanged Indications For Cesarean Sections: Ylva Vladic Stjernholm, Karin Petersson & Eva EnerothSam Ath SanNo ratings yet
- Vaginal Delivery of A Quadruplet Pregnancy Following Spontaneous ConceptionDocument5 pagesVaginal Delivery of A Quadruplet Pregnancy Following Spontaneous Conceptionzaheer ahmadNo ratings yet
- Perinatal Mortality Remains Very High in Monoamniotic Twin PregnanciesDocument6 pagesPerinatal Mortality Remains Very High in Monoamniotic Twin PregnanciesNovd NovitaNo ratings yet
- The Journal of Maternal-Fetal & Neonatal Medicine: To Cite This ArticleDocument8 pagesThe Journal of Maternal-Fetal & Neonatal Medicine: To Cite This ArticleBerry BancinNo ratings yet
- Case Reports in Women's Health: Olivia Dziadek, Paul SinghDocument3 pagesCase Reports in Women's Health: Olivia Dziadek, Paul SinghdeagratiaNo ratings yet
- Alobar HoloprosencephalyDocument3 pagesAlobar HoloprosencephalyRicky SilaenNo ratings yet
- Cs 2011Document7 pagesCs 2011listyaNo ratings yet
- Acta Obstet Gynecol Scand - 2011 - STJERNHOLM - Changed Indications For Cesarean SectionsDocument5 pagesActa Obstet Gynecol Scand - 2011 - STJERNHOLM - Changed Indications For Cesarean SectionsAli QuwarahNo ratings yet
- Brmedj00008 0063cDocument1 pageBrmedj00008 0063cNahrisyah Ulfa SafnaNo ratings yet
- Journal Pmed 1001208Document16 pagesJournal Pmed 1001208Carlos Emerson Rodriguez MalaverNo ratings yet
- Derksen Et Al-2004-Arthritis & Rheumatism PDFDocument12 pagesDerksen Et Al-2004-Arthritis & Rheumatism PDFAzwin Abdul JabbarNo ratings yet
- Guide to Pediatric Urology and Surgery in Clinical PracticeFrom EverandGuide to Pediatric Urology and Surgery in Clinical PracticeNo ratings yet
- Central Nervous System Tumours of ChildhoodFrom EverandCentral Nervous System Tumours of ChildhoodEdward EstlinNo ratings yet
- Stroke and Cerebrovascular Disease in ChildhoodFrom EverandStroke and Cerebrovascular Disease in ChildhoodVijeya GanesanNo ratings yet
- Diagnosis of Endometrial Biopsies and Curettings: A Practical ApproachFrom EverandDiagnosis of Endometrial Biopsies and Curettings: A Practical ApproachNo ratings yet
- Proteins: From DNA to StructureDocument58 pagesProteins: From DNA to StructuresujsamNo ratings yet
- KEY - Experimetnal ScenariosDocument2 pagesKEY - Experimetnal ScenariosArabellaNo ratings yet
- What Is Nervous System?: Presented By: TIP Grade 12 StudentDocument28 pagesWhat Is Nervous System?: Presented By: TIP Grade 12 StudentDreiza Patria SunodanNo ratings yet
- Control of A Robotic Arm Using ECGDocument48 pagesControl of A Robotic Arm Using ECGAnaNo ratings yet
- Worksheet in General Biology 2: Quarter 1 - Week 3: Theories of Evolution and The Development of Evolutionary ThoughtDocument10 pagesWorksheet in General Biology 2: Quarter 1 - Week 3: Theories of Evolution and The Development of Evolutionary ThoughtShastine ClaorNo ratings yet
- BacteriologyDocument14 pagesBacteriologyFrancene Ac-DaNo ratings yet
- 2011immunopathobiology of SyphilisDocument29 pages2011immunopathobiology of SyphilisStefani SardjonoNo ratings yet
- Everything You Need to Know About Choosing and Caring for Live Aquarium PlantsDocument8 pagesEverything You Need to Know About Choosing and Caring for Live Aquarium PlantsItz Adeyinka DanielNo ratings yet
- Polimiositis y Dermatomiositis FisiopatologiaDocument13 pagesPolimiositis y Dermatomiositis FisiopatologiaMargarita ChavezNo ratings yet
- 1.02 General Pathology - Cellular Pathology (Part 2) - Dr. Abelardo AleraDocument7 pages1.02 General Pathology - Cellular Pathology (Part 2) - Dr. Abelardo AleraCherry RahimaNo ratings yet
- Role of Intravenous Amino Acid Infusion in Cases of Oligohydramnios and Its Effect On Amniotic Fluid Index and Fetal Weight GainDocument6 pagesRole of Intravenous Amino Acid Infusion in Cases of Oligohydramnios and Its Effect On Amniotic Fluid Index and Fetal Weight GainRahimshaikhNo ratings yet
- Artificial Neural NetworkDocument9 pagesArtificial Neural Networkjyoti singhNo ratings yet
- Normal PeriodontiumDocument37 pagesNormal PeriodontiumEslam KillineyNo ratings yet
- Microbial Ecology and Microbial BiotechnologyDocument4 pagesMicrobial Ecology and Microbial BiotechnologynmdconahapNo ratings yet
- Dental BooklistDocument14 pagesDental Booklistالمكتبة المليونيةNo ratings yet
- Gustafsson 2017Document443 pagesGustafsson 2017Shennovy MarllonNo ratings yet
- Unit h420 01 Biological Processes Sample Assessment MaterialsDocument48 pagesUnit h420 01 Biological Processes Sample Assessment MaterialsGozde Ozan BayraktarNo ratings yet
- Bonne Pratique D'hygiéne PoissonDocument147 pagesBonne Pratique D'hygiéne PoissonLaila HomeNo ratings yet
- Maternal and Child Health ReferencesDocument4 pagesMaternal and Child Health ReferencesMeyke PotutuNo ratings yet
- List of Questions - HeredityDocument7 pagesList of Questions - Heredityshivani khareNo ratings yet
- Multiple-Choice Test: 3 EnzymesDocument5 pagesMultiple-Choice Test: 3 EnzymesMuhammadNo ratings yet
- Our Origins Discovering Physical Anthropology 3rd Edition Larsen Test BankDocument11 pagesOur Origins Discovering Physical Anthropology 3rd Edition Larsen Test Bankmrericsnowbqygmoctxk100% (14)
- Anti-H PyloripaperDocument10 pagesAnti-H PyloripaperLeandro DouglasNo ratings yet
- Pengajuan Alat UlumDocument2 pagesPengajuan Alat UlumAstrieLinglingNazrieliaNo ratings yet
- Laser Surface Treatment of Bio-Implant MaterialsDocument12 pagesLaser Surface Treatment of Bio-Implant MaterialsThe GantengNo ratings yet