Professional Documents
Culture Documents
Congenital Asymptomatic Diaphragmatic Hernias in Adults: A Case Series
Uploaded by
Rangga Duo RamadanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Congenital Asymptomatic Diaphragmatic Hernias in Adults: A Case Series
Uploaded by
Rangga Duo RamadanCopyright:
Available Formats
Bianchi et al.
Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
CASE REPORT
JOURNAL OF MEDICAL
CASE REPORTS
Open Access
Congenital asymptomatic diaphragmatic hernias
in adults: a case series
Enrica Bianchi1, Paola Mancini2, Stefania De Vito3, Elena Pompili3*, Samanta Taurone1, Isabella Guerrisi4,
Antonino Guerrisi4, Vito DAndrea5, Vito Cantisani5 and Marco Artico1
Abstract
Introduction: Congenital diaphragmatic hernia is a major malformation occasionally found in newborns and
babies. Congenital diaphragmatic hernia is defined by the presence of an orifice in the diaphragm, more often to
the left and posterolateral, that permits the herniation of abdominal contents into the thorax. The aim of this case
series is to provide information on the presentation, diagnosis and outcome of three patients with late-presenting
congenital diaphragmatic hernias. The diagnosis of congenital diaphragmatic hernia is based on clinical
investigation and is confirmed by plain X-ray films and computed tomography scans.
Case presentations: In the present report three cases of asymptomatic abdominal viscera herniation within the
thorax are described. The first case concerns herniation of some loops of the large intestine into the left hemithorax in a 75-year-old Caucasian Italian woman. The second case concerns a rare type of herniation in the right
side of the thorax of the right kidney with a part of the liver parenchyma in a 57-year-old Caucasian Italian woman.
The third case concerns herniation of the stomach and bowel into the left side of the chest with compression of
the left lung in a 32-year-old Caucasian Italian man. This type of hernia may appear later in life, because of
concomitant respiratory or gastrointestinal disease, or it may be an incidental finding in asymptomatic adults, such
as in the three cases featured here.
Conclusions: Patients who present with late diaphragmatic hernias complain of a wide variety of symptoms, and
diagnosis may be difficult. Additional investigation and research appear necessary to better explain the
development and progression of this type of disease.
Keywords: Congenital diaphragmatic hernia, CT scan, Diaphragmatic malformation, Plain X-ray films, Surgery
Introduction
Congenital diaphragmatic hernia (CDH) is an idiopathic
human malformation that usually presents in the newborn period. CDH is a relatively common condition that
occurs in less than one to five babies per 1000 births [1].
It seems to be slightly more frequent in men and less
frequent in Blacks [2,3]. This disease may be detected
during fetal life when screening ultrasonography demonstrates herniation of the bowel or liver into the thorax
and permits a correct pre-natal diagnosis in 50 percent
to 90 percent of cases. Polyhydramnios may lead to
antenatal diagnosis in some severe cases [4]. The onset
of CDH is generally due to respiratory distress or
* Correspondence: elena.pompili@uniroma1.it
3
Department of Anatomical, Histological, Forensic and Locomotor System
Sciences, V. A. Borelli 50, Rome 00161, Italy
Full list of author information is available at the end of the article
gastrointestinal disease, because of obstruction and incarceration of herniated bowel loops. Respiratory insufficiency is often secondary to pulmonary hypoplasia and
persistent pulmonary hypertension. The cause of pulmonary anomalies is discussed in the literature [5,6] and
seems to be caused by intra-thoracic compression of the
lungs by the herniated abdominal viscera, leading to alterations of pulmonary blood flow. Pulmonary hypoplasia in CDH is not just the result of direct compression
of lung parenchyma, because pulmonary development is
disrupted early in gestation before visceral herniation
has occurred. However, the etiology of pulmonary hypoplasia in CDH remains unknown. Respiratory and cardiovascular functions are severely compromised at birth
and this, together with the frequently associated
malformations, cause considerable rates of mortality and
morbidity. CDH should be included in the differential
2013 Bianchi et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative
Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work is properly cited.
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
diagnosis with pneumonia and other respiratory or
gastrointestinal diseases [7,8]. The neonatal symptoms of
CDH are heralded by respiratory distress with insufficient oxygenation, excavated abdomen with sternal protrusion and displacement of the heart sounds to the
contralateral side. The symptoms of insufficient gas exchange are associated with those of persistent pulmonary
hypertension [9,10] caused by arteriolar constriction and
closure of the pulmonary arterial bed that forces maintenance of a pattern of persistent fetal circulation in
which the blood from the right ventricle is shunted to
the left heart, preventing effective gas exchange. The
manifestations of CDH may occur at any age as mild respiratory distress or may even be an unexpected finding
during a medical check-up for other reasons [11]. In
these cases, a hernial sac is most often present [12].
Other organs may be involved in CDH [13] because associated malformations are frequent [14].
The heart and vessels are often abnormal in patients
with CDH. A diagnosis of CDH is unexpected if the patient is asymptomatic. In such cases radiological findings
play a predominant role. The radiological features on
X-ray films are not always easy to detect. Typically,
radiological images show intra-thoracic gas-filled loops
of the bowel with a contralateral shift of the mediastinum. In some cases, the herniated bowel loops may be
complicated, such as in obstruction or volvulus, and the
fluid-filled bowel loops appear as an opacity mimicking
a lung consolidation. In other cases the gas-filled bowel
loops may simulate a pneumothorax [15,16].
A computed tomography (CT) scan is the radiological
investigation that allows the highest accuracy for a correct diagnosis. It provides a precise assessment of the
anatomical relationships between the viscera, and congenital malformations, as in our cases.
Very few cases of delayed presentation of CDH in
asymptomatic adults are described in the literature. We
report three cases in adult patients, presenting with herniation of the abdominal viscera in the thorax, who
underwent CT scans for symptoms not related to either
herniation or respiratory disease. In one of these cases, a
rare occurrence of right abdomen viscera herniated into
the right hemi-thorax was observed.
Case presentations
Case 1
A 75-year-old Caucasian Italian woman with hypereosinophilia underwent a CT scan to evaluate her lung parenchyma. The eosinophilia was diagnosed as idiopathic.
No respiratory symptoms were reported by our patient,
or any symptoms related to gastrointestinal disease. Our
patient agreed to a CT scan after signing the informed
consent. Our patient underwent a CT scan without
intravenous contrast administration. The mediastinum
Page 2 of 8
window level showed a small pleural fluid collection on
the left side of the thorax and a few small lymph nodes
around the trachea (Figure 1). No focal or diffuse lung
parenchyma pathological involvement was found. The
esophagus was correctly positioned on the right side of
the descending aorta, besides the trachea. Posteriorly to
the tracheal bifurcation, gas-filled large bowel loops were
evident (Figure 1), located in the posterior mediastinum,
anterior to the esophagus and the descending aorta. The
bowel loops occupied the left inferior hemi-thorax, near
the left heart ventricle. The other abdominal viscera,
such as the spleen, stomach and the rest of the bowel
loops, were situated in their correct anatomical positions. Our patient did not receive surgical treatment because she was asymptomatic.
Case 2
A 57-year-old Caucasian Italian woman with right heart
overflow underwent a CT scan with intravenous contrast
administration. Our patient agreed to a CT scan after
signing the informed consent. The mediastinum window
level was performed in the venous phase after the injection of contrast medium. The CT showed some anomalies of the vascular structures: the inferior vena cava was
enlarged from its retro-hepatic segment as far as the
efflux in the right atrium, which also appeared mildly
enlarged (Figure 2). The pulmonary vessels of the right
hemi-thorax, as well as the right pulmonary artery,
appeared to be increased in diameter, as in a blood overflow condition. The left pulmonary vessels were normal.
CT scans showed the superior portion of the right kidney and a part of the liver parenchyma (which may be
ascribed to the VI hepatic segment) occupying the right
basal hemi-thorax. The middle and inferior part of the
kidney remained in the abdomen, with the right adrenal
gland and the rest of the renal pelvis (Figure 2). The
right renal parenchyma appeared slightly larger than the
contralateral one, but the renal pelvis and the ureter
were normal. Renal function appeared regular, with normal elimination of the contrast agent bilaterally. The left
kidney was in a slightly higher position in the abdomen,
contiguous to the spleen, in the abdominal cavity. Surgical repair with a tension-free patch of Gore-Tex was
proposed to our patient, but she refused surgery because
of the possible post-operative risks and complications;
she was subsequently discharged.
Case 3
A 32-year-old Caucasian Italian man with hypereosinophilia underwent a CT scan to evaluate his lung parenchyma. The eosinophilia was diagnosed as idiopathic.
Our patient complained of occasional vomiting, nausea,
epigastric pain and palpitations. Respiratory sounds were
found to be diminished at the left basal region on
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
Page 3 of 8
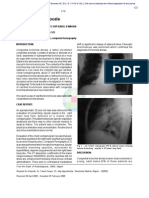
Figure 1 (A,B) Axial CT scan of the chest. Gas-filled large bowel loops are visible behind the heart, laying anteriorly to the spine and the aorta,
with part of the abdominal fat.
auscultation, where occasional peristaltic sounds were
appreciable. Our patient agreed to a CT scan after
signing the informed consent. Our patient underwent
a CT scan without intravenous contrast administration (Figure 3A,B), which demonstrated herniation of
the stomach (adjacent to the heart, Figure 3A) and of
bowel into the left side of the chest with compression
of the left lung (Figure 3B). Surgical repair with a
tension-free patch of Gore-Tex was proposed to our
patient, but he refused surgery because of his scarce
and intermittent symptoms and was subsequently
discharged.
Discussion
The diaphragm divides the thorax from the abdomen
and is derived from several sources during embryological
development, mainly the septum transversum. This is
situated at first rostrally to the somites in the embryonic
period and acts as a mesodermal bridge between the
pericardial and the umbilical vesicle cavities. Its cranial
portion becomes covered by pericardium and pleura,
whereas its ventral part is a sagittal mesentery that
contains the expanding liver. The septum transversum
gives rise to the majority of the diaphragm and descends
from the cervical to thoracic level during embryonic
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
Figure 2 Computed tomography scan. Part of the right kidney
and an enlarged inferior vena cava are shown, flowing into the also
enlarged right atrium.
Page 4 of 8
development. Normal diaphragm closure occurs at the
eighth week.
The other parts of the diaphragm consist of the
pleuroperitoneal membranes and the mesenchyma of
the body wall. The pleuroperitoneal canals may be
closed by the contribution of the subjacent organs, such
as the liver and the suprarenal glands. The skeletal
muscle of the diaphragm is probably derived in situ from
the body wall and also through the migration from cervical myotomes.
The CDH is a displacement of the abdominal organs
into the thoracic cavity through a weak area or a distinct
defect in the diaphragm. The causes of precipitating
viscera herniation may be related to mechanical or pressure changes in the thoracoabdominal cavities. The most
frequent types of diaphragmatic hernia are the left posterolateral (Bochdalek hernia) and the sternocostal
(Morgagni hernia) types. A Bochdalek hernia, resulting
from inadequate closure of the posterolateral pleuroperitoneal membrane, is the most frequently seen
congenital diaphragmatic hernia. Defects occur more
frequently on the left side than on the right side of the
Figure 3 (A,B) Computed tomography scan of the chest. Coronal reconstruction. Part of the stomach, adjacent to the heart (A), is clearly
visible. Some bowel loops (B) are also visible on left side of the chest: the left lung is displaced and compressed.
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
diaphragm, and the abdominal contents, including stomach, bowel loops, liver, spleen or fat tissues, may be
displaced into the thoracic cavity. A posterolateral hernia
on the right side is very rare and this is probably attributable to the protection provided by the liver. Foramen
of Morgagni hernias are rare diaphragmatic hernias,
usually occurring on the right and located in the anterior
mediastinum because of the retrosternal location of the
foramen of Morgagni, described as an anterior diaphragmatic defect. In adults, foramen of Morgagni hernia is
also associated with obesity, trauma, weight lifting, or
other causes of increased intra-abdominal pressure.
The most frequent cause of herniation of the abdominal viscera in adults seems to be trauma, whereas in babies or newborns it is most often attributable to
congenital absence or defective fusion of the septum
transversum or the pleuroperitoneal membrane. The detection of diaphragmatic hernia is made with pre-natal
ultrasonography in 50 percent to 90 percent of cases
[17,18]. The intestine and the liver may be in the thorax
and the lungs are small. Ultrasound scans allow detailed
assessment of the heart. Lung growth is measured as a
proportion of head growth. The lung-to-head ratio
(LHR) has some prognostic value [19-25], because when
it is below 1, survival is compromised [26]. When diagnosis is made in utero, amniocentesis is often performed
for detecting chromosomal aberrations [27] and may
help to estimate lung maturity [28]. After birth, a diagnosis can readily be made on the basis of symptoms and
physical signs. A plain X-ray of the thorax and abdomen
provides details of the position of the herniated viscera.
Blood gases and pH status reflect the efficiency of gas
exchange. A physical examination may be sufficient, but
passing a naso-gastric catheter into the stomach before a
plain X-ray of the thorax and abdomen may help to locate it or to detect any esophageal displacement [29]. In
some rare cases, herniation of viscera through the diaphragm is an incidental finding in adult patients undergoing plain X-ray films or CT scans for other symptoms
not related to this pathology. Since our three adult patients had asymptomatic or poorly symptomatic diaphragmatic hernia we did not refer the herniation to any
traumatic event and so the diaphragmatic defects were,
presumably, congenital. A large proportion of fetuses
with CDH are diagnosed in utero and termination of
gestation is sometimes preferred, particularly when
chromosomal aberrations and syndromes are present
[30-33]. The possibility of fetal instrumentation directed
to alleviate the consequences of the herniation is
becoming a progressively more acceptable alternative.
The current development of minimally invasive surgery
has brought about the immediate effect of making
fetendoscopic balloon tracheal occlusion possible in
fetuses with LHR below 1 [34]. Experimental and clinical
Page 5 of 8
evidence suggest that biochemical lung maturation is
delayed in fetuses with CDH [35] and this led to the
assumption that this process is accelerated by administration of maternal corticosteroids as carried out for premature deliveries. However, since the evidence of the
benefits of this medication are not totally convincing
[36], a multicenter trial could be useful [37].
Whenever pre-natal diagnosis is made, gestation
should be prolonged until near term if possible [38] and
there is no evidence of the benefits of delivery by
Caesarean section [38]. Treatment after birth requires
extra-corporeal membrane oxygenation (ECMO) prior
to surgical correction. Extra-corporeal membrane oxygenation can be seen as a safety net maintained until
proper gas exchange occurs. Cannulation of both the
right carotid artery and jugular vein and connection to a
circuit with a membrane gas exchange chamber allows
oxygenation and CO2 disposal without participation of
the lung, which is thus preserved from any pressure insult [39]. This technique is probably a good adjunct in a
limited number of patients in whom predicted severe
lung hypoplasia would make adequate gas exchange impossible or in those in whom reversal to the fetal pattern
of circulation becomes unmanageable. In the past, surgical repair of CDH used to be a life-saving emergency
procedure. It is presently accepted that this procedure
should be undertaken only once cardio-respiratory functions have been stabilized. A policy of delayed surgery
coupled with gentle ventilation and occasional ECMO
support has yielded the best results to date. The goal
of all forms of pre-operative treatment is to obtain
stabilization of the patient: this requires acceptable oxygenation and CO2 disposal with stable pulmonary pressures, tolerable shunting, good myocardial function and
adequate renal clearance with reduced or withdrawn
inotropic drugs. Under general anesthesia, a subcostal or
transverse abdominal incision is made and the herniated
viscera are carefully reduced into the abdomen: the diaphragmatic orifice is closed with interrupted sutures
without leaving an intercostal tube. A tube was routinely
used in the past [40] until it was realized that underwater seals cause increased respiratory work and
overdistension of the hypoplastic lung that may reduce
ventilation even further. A tubeless policy was then advised [41]. When the defect is too large, a prosthetic
patch is used to achieve closure [42]. It is sutured to the
rims of the orifice with interrupted sutures and, to avoid
excessive tension and enlargement of the hemi-thorax,
cone shaping of the patch can be beneficial [43]. The use
of a patch seems to increase the risk of re-herniation
[44] although it should be acknowledged that patients
requiring a patch have larger defects that entail higher
morbidity [45]. Abdominal wall or dorsi muscle flaps
have also been used for CDH repair [46]. Since the
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
advent of minimally invasive surgery (MIS) thoracoscopic [47] or laparoscopic [48] approaches have been
proposed for CDH repair. MIS is a good approach in
cases diagnosed during infancy with less severe symptoms. Regardless, some concerns about this particular
approach in the newborn period have been expressed on
the basis of excessive peri-operative hypercapnia and
prolonged post-operative low brain oxygenation. The
risk of recurrence after a minimally invasive approach
has been found to be similar to that of open surgery.
Congenital diaphragmatic hernias mainly present in
the neonatal period and are associated with a mortality
that has not changed much despite the advances made
in critical care. Rarely, these hernias present later in life,
some even in adulthood. There are numerous reports of
CDH presenting after infancy. These patients are either
asymptomatic or have minimal respiratory symptoms,
possibly because the lungs are not hypoplastic.
Late-presenting CDH is often difficult to diagnose, and
delays in treatment are common. All diaphragmatic
defects could be closed by an abdominal approach without post-operative complications. Clinical symptoms
disappeared post-operatively. In adulthood a CDH may
present with gastrointestinal tract symptoms that may
include intermittent abdominal pain, vomiting, and dysphagia. Respiratory symptoms usually include dyspnea
and chest pain [49]. Symptoms may be intermittent or
acute depending on the extent of herniation of abdominal viscera into the thorax. An acute presentation is
usually due to incarceration, obstruction, or strangulation of the herniated viscera [50]. Diagnosis is
ascertained by a combination of chest X-rays, CT and
magnetic resonance imaging (MRI), as well as upper
gastrointestinal and bowel double-contrast studies.
Typical findings on a CT scan would be the abutment of
fat or soft tissue along the upper surface of the diaphragm, characteristic posterolateral location on the
hemidiaphragm, diaphragmatic discontinuity adjacent to
the mass, and continuous density above and below the
diaphragm through the defect [51]. Bochdalek hernia
may be misdiagnosed as pleural effusion, pneumonia,
tension pneumothorax, lung cysts, or atelectasis.
A careful analysis of chest films and a thorough search
for connecting bowel segments passing through the diaphragmatic defect may help to avoid incorrect diagnosis
and an undesirable delay in treatment. Confusion with
pneumonia or pneumothorax can be diminished by placing a feeding tube and instillation of contrast material.
Management of a Bochdalek hernia includes reducing
the abdominal contents and repairing the defect through
a laparotomy or thoracotomy. Successful laparoscopic
and thoracoscopic repairs of Bochdalek hernias have
both been described. Right-sided defects are usually
dealt with by a thoracic or thoracoabdominal approach
Page 6 of 8
because of the presence of the liver. For left-sided hernias some authors suggest a transthoracic approach
while others advise a transperitoneal approach [52]. The
indications for repair of congenital diaphragmatic hernia
are the same as those to treat any hernia, and should
take into account the patients overall medical condition.
The outcome of adult patients suffering from Bochdalek
hernia depends on the type of clinical presentation.
Delay in the diagnosis of CDH can result in significant
morbidity [53].
Conclusions
The cases reported in our study are delayed presentations of CDH in a posterior location (Bochdaleks-like
viscera herniation) detected as an incidental finding on
chest CT scans.
In the first case there was herniation of the large
bowel on the posterior side of the mediastinum. The
second case appeared to be more unusual because the
viscera were herniated through a defect on the right side
of the diaphragm in the right hemithorax. Moreover, in
the second case the herniated viscera exerted compression on the right side of the heart, resulting in a blood
overflow in the pulmonary circulation and a small
pleural fluid collection on the left side of the thorax. In
the third case, there was herniation of the upper part of
the stomach into the thorax through a tear or weakness
in the diaphragm. In such cases, where hernia symptoms
are severe and chronic acid reflux is involved, surgery is
sometimes recommended, since chronic reflux can severely injure the esophagus and even lead to esophageal
cancer. Generally speaking, plain X-ray films may be
considered the primary radiological investigation able to
show the presence of gas-filled bowel loops within the
thoracic cavity. However, in our study, our patients initially underwent chest CT scans because this investigation facilitates diagnosis of such anatomical variations
and establishes the relationships between the abnormally
positioned viscera.
We report three cases of delayed presentation of a potentially life-threatening CDH. The variable clinical features of CDH presenting beyond the neonatal period may
result in clinical and radiological misdiagnosis. CDH with
complicating mediastinal shift and respiratory distress requires urgent gastrointestinal decompression and respiratory support. The most significant factor in achieving
diagnostic success is to consider it early in the differential
diagnosis to avoid misguided or delayed therapy.
In conclusion, CDH is a complex condition probably
caused by disturbed molecular signaling during
organogenesis. The diaphragmatic orifice is invariably
accompanied by pulmonary hypoplasia with vascular
hyper-reactivity that causes deficient gas exchange and
persistent pulmonary hypertension. In addition, other
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
malformations that further complicate the clinical course
may be present. Regardless, many aspects of the disease
are still unknown and, given that the incidence is relatively high, that the expenses involved in the current
treatments are overwhelming and that the sequelae are
frequent, more research efforts into causation, prevention and treatment are warranted.
Consent
Written informed consent was obtained from the patients
for publication of this manuscript and any accompanying
images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Authors contributions
EB, EP, PM, ST, SDV, IG, AG, VDA and VC analyzed and interpreted the data
from our patients regarding radiological investigation by plain X-ray films
and CT scans. EB and MA were the main contributors in writing the
manuscript. All authors read and approved the final manuscript.
Author details
1
Department of Sensory Organs, University Sapienza, Rome, Italy.
2
Department of Radiology, S. Giovanni Calibita Isola Tiberina, Fatebenefratelli
Hospital, Rome, Italy. 3Department of Anatomical, Histological, Forensic and
Locomotor System Sciences, V. A. Borelli 50, Rome 00161, Italy. 4Department
of Radiological Sciences, University of Rome Sapienza, Rome 00159, Italy.
5
Department of Radiology, Oncology and Pathology, University La Sapienza,
Policlinico Umberto I, Rome, Italy.
Received: 27 August 2012 Accepted: 5 March 2013
Published: 13 May 2013
References
1. Gattot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C,
Laurichesse-Delmas H, Jani J, Coste K, Deprest J, Labbe A, Sapin V, Lemery
D: Prenatal detection and outcome of congenital diaphragmatic hernia:
a French registry-based study. Ultrasound Obstet Gynecol 2007, 29:276283.
2. Torfs CP, Curry CJ, Bateson TF, Honore LH: A population-based study of
congenital diaphragmatic hernia. Teratology 1992, 46:555565.
3. Yang W, Carmichael SL, Harris JA, Shaw GM: Epidermiologic characteristics
of congenital diaphragmatic hernia among 2.5 million California births,
19891997. Birth Defects Res A Clin Mol Teratol 2006, 76:170174.
4. Sinha CK, Islam S, Patel S, Nicolaides K, Greenough A, Devenport M:
Congenital diaphragmatic hernia: prognostic indices in the fetal
endoluminal tracheal occlusion era. J Pediatr Surg 2009, 44:312316.
5. Bronshtein M, Lewit N, Sujov PO, Markoul IR, Blazer S: Prenatal diagnosis of
congenital diaphragmatic hernia: timing of visceral herniation and
outcome. Pren Diagn 1995, 15:695698.
6. Jesudason EC: Challenging embryological theories on congenital
diaphragmatic hernia: future therapeutic implications for paediatric
surgery. Ann R Coll Surg Engl 2002, 84:252259.
7. Berman L, Stringer DA, Ein S, Shandling B: Childhood diaphragmatic hernias
presenting after the neonatal period. Clin Radiol 1988, 39:237244.
8. Fotter R, Schimpl G, Sorantin E, Fritz K, Landler U: Delayed presentation of
congenital diaphragmatic hernia. Clin Radiol 1992, 22:187191.
9. de Buys Roessingh AS, Dinh-Xuan AT: Congenital diaphragmatic hernia:
current status and review of the literature. Eur J Pediatr 2009, 168:393406.
10. Keijzer R, Puri P: Congenital diaphragmatic hernia. Semin Pediatr Surg 2010,
19:180185.
11. Elhalaby EA, Abo Sikeena MH: Delayed presentation of congenital
diaphragmatic hernia. Pediatr Surg Int 2002, 18:480485.
12. Numamoglu A, Steiner Z, Millar A, Cywes S: Delayed presentation of
congenital diaphragmatic hernia. S Afr J Surg 1997, 35:7476.
Page 7 of 8
13. Stoll C, Alembik Y, Dott B, Roth MP: Associated malformations in cases
with congenital diaphragmatic hernia. Genet Couns 2008, 19:331339.
14. Sweed Y, Puri P: Congenital diaphragmatic hernia: influence of associated
malformations on survival. Arch Dis Child 1993, 69:6870.
15. Lin ST, Moss DM, Henderson SO: A case of Morgagni hernia presenting as
pneumonia. J Em Med 1997, 15:297301.
16. Gayer G, Bilik R, Vardi A: CT diagnosis of delayed presentation of
congenital diaphragmatic hernia simulating massive pleuropneumonia.
Eur Radiol 1999, 9:16721674.
17. Nakayama DK, Harrison MR, Chrinn DH, Callen PW, Filly RA, Golbus MS, De
Lorimier AA: Prenatal diagnosis and natural history of the fetus with a
congenital diaphragmatic hernia: initial clinical experience. J Pediatr Surg
1985, 20:118124.
18. Adzick NS, Vacanti JP, Lillehei CW, ORourke PP, Crone RK, Wilson JM: Fetal
diaphragmatic hernia: ultrasound diagnosis and clinical outcome in 38
cases. J Pediatr Surg 1989, 24:654657.
19. Knox E, Lissauer D, Khan K, Kilby M: Prenatal detection of pulmonary
hypoplasia in fetuses with congenital diaphragmatic hernia: a systematic
review and meta-analysis of diagnostic studies. J Matern Fetal Neonatal
Med 2010, 23:579588.
20. Sandaite I, Claus F, De Keyzer F, Done E, Van Mieghem T, Guacciardo L,
Dekoninck P, Jani J, Cannie M, Deprest JA: Examining the relationship
between the lung-to-head ratio measured on ultrasound and lung
volumetry by magnetic resonance in fetuses with isolated congenital
diaphragmatic hernia. Fetal Diagn Ther 2011, 29:8087.
21. Harrison MR, Sydorak RM, Farrell JA, Kitterman JA, Filly RA, Albanese CT:
Fetoscopic temporary tracheal occlusion for congenital diaphragmatic
hernia: prelude to a randomized, controlled trial. J Pediatr Surg 2003,
38:10121020.
22. Jani J, Peralta CF, Van Schoubroeck D, Deprest J, Nicolaides KH:
Relationship between lung-to-head ratio and lung volume in normal
fetuses and fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol
2006, 27:545550.
23. Usui N, Okuyama H, Sawai T, Kamata S, Fukuzawa M: Relatioship between
L/T ratio and LHR in the prenatal assessment of pulmonary hypoplasia
in congenital diaphragmatic hernia. Pediatr Surg Int 2007, 23:971976.
24. Jani JC, Benachi A, Nicolaides KH, Allegaert K, Graracos E, Mazkereth R, Matis
J, Tibboel D, Van Heijst A, Storme L, Rousseau V, Greenough A, Deprest JA:
Prenatal prediction of neonatal morbidity in survivors with congenital
diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol
2009, 33:6469.
25. Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS: Sonographic
predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 1996,
31:148151.
26. Lipshutz GS, Albanese CT, Feldstein VA, Jennings RW, Housley HT, Beech R,
Farrell JA, Harrison MR: Prospective analysis of lung-to-head ratio predicts
survival for patients with prenatally diagnosed congenital diaphragmatic
hernia. J Pediatr Surg 1997, 32:16341636.
27. Takahashi H, Hayashi S, Miura Y, Tsukamoto K, Kosaki R, Itoh Y, Sago H:
Trisomy 9 mosaicism diagnosed in utero. Obstet Gynecol Int 2010.
pii:379534.
28. Moya FR, Thomas VL, Romaguera J, Mysore MR, Maberry M, Bernard A,
Freund M: Fetal lung maturation in congenital diaphragmatic hernia.
Am J Obstet Gynecol 1995, 173:14011405.
29. Tovar JA: Congenital diaphragmatic hernia. Orphanet J Rare Dis 2012,
7:115.
30. Stege G, Fenton A, Jaffray B: Nihilism in the 1990s: the true mortality of
congenital diaphragmatic hernia. Pediatrics 2003, 112:532535.
31. Garne E, Haeusler M, Barisic I, Gjergja R, Stoll C, Clementi M: Congenital
diaphragmatic hernia: evaluation of prenatal diagnosis in 20 European
regions. Ultrasound Obstet Gynecol 2002, 19:329333.
32. Colvin J, Bower C, Dickinson JE, Sokol J: Outcomes of congenital
diaphragmatic hernia: a population-based study in Western Australia.
Pediatrics 2005, 116:e356e363.
33. Richmond S, Atkins J: A population-based study of the prenatal diagnosis
of congenital malformation over 16 years. BJOG 2005, 112:13491357.
34. Deprest JA, Lerut TE, Vandenberghe K: Operative fetoscopy: new
perspective in fetal therapy? Prenat Diagn 1997, 17:12471260.
35. Valls-i-Soler A, Alfonso LF, Arnaiz A, Alvarez FJ, Tovar JA: Pulmonary
surfactant dysfunction in congenital diaphragmatic hernia: experimental
and clinical findings. Biol Neonate 1996, 69:318326.
Bianchi et al. Journal of Medical Case Reports 2013, 7:125
http://www.jmedicalcasereports.com/content/7/1/125
Page 8 of 8
36. Lally KP, Bagolan P, Hosie S, Lally PA, Stewart M, Cotten CM, Van Meurs KP,
Alexander G: Corticosteroids for fetuses with congenital diaphragmatic
hernia: can we show benefit? J Pediatr Surg 2006, 41:668674.
37. Keijzer R, van Tuyl M, Tibboel D: Hormonal modulation of fetal pulmonary
development: relevance for the fetus with diaphragmatic hernia.
Eur J Obstet Gynecol Reprod Biol 2000, 92:127133.
38. Stevens TP, van Wijngaarden E, Ackerman KG, Lally PA, Lally KP: Timing of
delivery and survival rates for infants with prenatal diagnoses of
congenital diaphragmatic hernia. Pediatrics 2009, 123:494502.
39. Bartlett RH, Gazzaniga AB, Toomasian J, Coran AG, Roloff D, Rucker R:
Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory
failure. 100 cases. Ann Surg 1986, 204:236245.
40. Clark RH, Hardin WD Jr, Hirschl RB, Jaksic T, Lally KP, Langham MR Jr, Wilson
JM: Current surgical management of congenital diaphragmatic hernia: a
report from the congenital diaphragmatic hernia study group. J Pediatr
Surg 1998, 33:10041009.
41. Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ: Congenital diaphragmatic
hernia: survival treated with very delayed surgery, spontaneous
respiration, and no chest tube. J Pediatr Surg 1995, 30:406409.
42. Fei L, Saviano C, Moccia F, del Genio G, Trapani V, Nunziale A, Lombardi G,
Cecchi M: ePTFE soft tissue patch reconstruction of hemidiaphragmatic
agenesis with late clinical presentation. Hernia 2008, 12:103106.
43. Loff S, Wirth H, Jester I, Hosie S, Wollmann C, Schaible T, Ataman O, Waag
KL: Implantation of a cone-shaped double-fixed patch increases
abdominal space and prevents recurrence of large defects in congenital
diaphragmatic hernia. J Pediatr Surg 2005, 40:17011705.
44. Atkinson JB, Poon MW: ECMO and the management of congenital
diaphragmatic hernia with large diaphragmatic defects requiring a
prosthetic patch. J Pediatr Surg 1992, 27:754756.
45. Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, Wilson JM, Frenckner B, Van
Meurs KP, Bohn DJ, Davis CF, Hirschl RB: Defect size determines survival in
infants with congenital diaphragmatic hernia. Pediatrics 2007, 120:e651e657.
46. Nasr A, Struijs MC, Ein SH, Langer JC, Chiu PP: Outcomes after muscle flap
vs prosthetic patch repair for large congenital diaphragmatic hernias.
J Pediatr Surg 2010, 45:151154.
47. Nguyen TL, Le AD: Thoracoscopic repair for congenital diaphragmatic
hernia: lessons from 45 cases. J Pediatr Surg 2006, 41:17131715.
48. Meehan JJ, Sandler A: Robotic repair of a Bochdalek congenital
diaphragmatic hernia in a small neonate: robotic advantages and
limitations. J Pediatr Surg 2007, 42:17571760.
49. Nitecki S, Bar-Maor JA: Late presentation of Bochdalek hernia: our
experience and review of the literature. Isr J Med Sci 1992, 28:711714.
50. Sridhar AV, Nichani S: Late presenting congenital diaphragmatic hernia.
Emerg Med J 2004, 21:261262.
51. Wilbur AC, Gorodetsky A, Hibbeln JF: Imaging findings of adult Bochdalek
hernias. Clin Imaging 1994, 18:224229.
52. Laaksonen E, Silvasti S, Hakala T: Right-sided Bochdalek hernia in an adult:
a case report. J Med Case Reports 2009, 3:9291.
53. Baerg J, Kanthimathinathan V, Gollin G: Late-presenting congenital
diaphragmatic hernia: diagnostic pitfalls and outcome. Hernia 2012,
16:461466.
doi:10.1186/1752-1947-7-125
Cite this article as: Bianchi et al.: Congenital asymptomatic
diaphragmatic hernias in adults: a case series. Journal of Medical Case
Reports 2013 7:125.
Submit your next manuscript to BioMed Central
and take full advantage of:
Convenient online submission
Thorough peer review
No space constraints or color gure charges
Immediate publication on acceptance
Inclusion in PubMed, CAS, Scopus and Google Scholar
Research which is freely available for redistribution
Submit your manuscript at
www.biomedcentral.com/submit
You might also like
- Self Healing Paida LajinDocument8 pagesSelf Healing Paida LajinPervaiz Ahmad100% (2)
- The Many Benefits of Hydrogen PeroxideDocument9 pagesThe Many Benefits of Hydrogen PeroxideRedza100% (7)
- Comparing Public and Self-Stigma in Mental IllnessDocument5 pagesComparing Public and Self-Stigma in Mental IllnessAlexandruIulianDorneanuNo ratings yet
- Simply Psych Cheat Sheets Dsm5 Part 1 LissaurDocument2 pagesSimply Psych Cheat Sheets Dsm5 Part 1 LissaurChhitiz Kiran ShresthaNo ratings yet
- Pediatricreferencecard 04Document2 pagesPediatricreferencecard 04yesumovsNo ratings yet
- Acid Group of Remedies in HomoeopathyDocument5 pagesAcid Group of Remedies in HomoeopathyNagender UpadhyayNo ratings yet
- DR Varsha Atul Shah Senior Consultant Dept of Neonatal and Devt Medicine, SGH Visiting Consultant Dept of Child Devt, KKHDocument47 pagesDR Varsha Atul Shah Senior Consultant Dept of Neonatal and Devt Medicine, SGH Visiting Consultant Dept of Child Devt, KKHJennifer MrjNo ratings yet
- Assessing Appearance and Mental StatusDocument12 pagesAssessing Appearance and Mental StatusAaron Perez SubidaNo ratings yet
- Pleural EffusionDocument10 pagesPleural EffusionShane PangilinanNo ratings yet
- Iloilo Chinese Commercial School V FabrigarDocument2 pagesIloilo Chinese Commercial School V FabrigarJohn Erik Kapunan100% (3)
- Congenital Diaphragmatic HerniasDocument20 pagesCongenital Diaphragmatic HerniasRahma Tya AnwarNo ratings yet
- Ruptured hepatic abscess mimicking perforated viscusDocument3 pagesRuptured hepatic abscess mimicking perforated viscusMichu VuNo ratings yet
- Congenital Diaphragmatic Hernia: Review Open AccessDocument15 pagesCongenital Diaphragmatic Hernia: Review Open AccessPutri YingNo ratings yet
- APENDICITIS Fischers Mastery of Surgery 7th ING PDFDocument33 pagesAPENDICITIS Fischers Mastery of Surgery 7th ING PDFMichel RamirezNo ratings yet
- Bladder Diverticulum and SepsisDocument4 pagesBladder Diverticulum and SepsisInternational Medical PublisherNo ratings yet
- Intralobar Pulmonary Sequestration: Incidental Finding in An Asymptomatic PatientDocument4 pagesIntralobar Pulmonary Sequestration: Incidental Finding in An Asymptomatic PatienteduardoNo ratings yet
- Asthma and Lung MassDocument4 pagesAsthma and Lung MassAzmachamberAzmacareNo ratings yet
- Perforated Ileal Diverticulitis: CT Findings: İsmail Kırbaş, Erkan Yıldırım, Ali Harman, Özgür BaşaranDocument2 pagesPerforated Ileal Diverticulitis: CT Findings: İsmail Kırbaş, Erkan Yıldırım, Ali Harman, Özgür BaşaranKelfin ForceNo ratings yet
- Congenital Diaphragmatic Hernia: Orphanet Journal of Rare Diseases January 2012Document16 pagesCongenital Diaphragmatic Hernia: Orphanet Journal of Rare Diseases January 2012danny anantaNo ratings yet
- Seear 2017Document8 pagesSeear 2017Lucia NiculaeNo ratings yet
- 19-22 Aortoenteric Fistula A Possible Cause of Sudden DeathDocument4 pages19-22 Aortoenteric Fistula A Possible Cause of Sudden DeathLoredana MorosanuNo ratings yet
- Recurrent Pneumonia Caused by Rare Granular Cell TumorDocument7 pagesRecurrent Pneumonia Caused by Rare Granular Cell TumorJoshua MendozaNo ratings yet
- Chilaiditi Syndrome - A Benign Air Under DiaphragmDocument1 pageChilaiditi Syndrome - A Benign Air Under DiaphragmBaran PalanimuthuNo ratings yet
- 500 FullDocument4 pages500 FullAina NurlailaNo ratings yet
- Penetrating Gunshot Injury To The Chest With Unusual Intraluminal Passage of The BulletDocument4 pagesPenetrating Gunshot Injury To The Chest With Unusual Intraluminal Passage of The BulletSummaiyahWahabKhanNo ratings yet
- The Anatomy and Diagnosis of AppendicitisDocument11 pagesThe Anatomy and Diagnosis of AppendicitisAnjung WibiksanaNo ratings yet
- AppendicitisDocument36 pagesAppendicitisPetro MyronovNo ratings yet
- Comparison of Transverse and Vertical Skin Incision ForDocument6 pagesComparison of Transverse and Vertical Skin Incision ForHerry SasukeNo ratings yet
- Liver Abscess DissertationDocument4 pagesLiver Abscess DissertationPayForAPaperAtlanta100% (1)
- LAPcarlberg 2016Document21 pagesLAPcarlberg 20164bm5hb2dydNo ratings yet
- Diaphragmatic Eventration Misdiagnosed As Diaphragmatic Hernia in A Preterm Infant With Respiratory Distress: A Case Report and Review of Diagnosis and ManagementDocument4 pagesDiaphragmatic Eventration Misdiagnosed As Diaphragmatic Hernia in A Preterm Infant With Respiratory Distress: A Case Report and Review of Diagnosis and ManagementPeertechz Publications Inc.No ratings yet
- Primary Aortoenteric Fistula Due to Septic Aortitis CaseDocument5 pagesPrimary Aortoenteric Fistula Due to Septic Aortitis CaseKintrili AthinaNo ratings yet
- 1 PBDocument9 pages1 PBMariiaNo ratings yet
- Jurnal 2Document0 pagesJurnal 2Seftiana SaftariNo ratings yet
- Early Diagnosis of Kartagener's SyndromeDocument8 pagesEarly Diagnosis of Kartagener's SyndromeHendra SusantoNo ratings yet
- RVOT Stenting in TOF PatientDocument31 pagesRVOT Stenting in TOF PatientHannaTashiaClaudiaNo ratings yet
- A Rare Case of Colon Cancer in A Young Patient: A Case Report and Literature ReviewDocument5 pagesA Rare Case of Colon Cancer in A Young Patient: A Case Report and Literature ReviewIJAR JOURNALNo ratings yet
- Gi 1Document3 pagesGi 1Syifa' FauziyahNo ratings yet
- Lung Cancer in ChildDocument4 pagesLung Cancer in Childtonirian99No ratings yet
- TAPVR in Neonate NeoReviews Jan2019Document3 pagesTAPVR in Neonate NeoReviews Jan2019Sridhar KaushikNo ratings yet
- Acute Appendicitis in Adults: Clinical Manifestations and DiagnosisDocument37 pagesAcute Appendicitis in Adults: Clinical Manifestations and DiagnosisDaniela MuñozNo ratings yet
- Case Report: Pulmonary Sequestration: A Case Report and Literature ReviewDocument4 pagesCase Report: Pulmonary Sequestration: A Case Report and Literature Reviewjo_jo_maniaNo ratings yet
- Indian J Radiol Imaging Word 1Document14 pagesIndian J Radiol Imaging Word 1Adie BrianNo ratings yet
- Simultaneous Bilateral Primary Spontaneous Pneumothorax (2019)Document7 pagesSimultaneous Bilateral Primary Spontaneous Pneumothorax (2019)sandi_prawira_yudhaNo ratings yet
- CDH Modern MXDocument11 pagesCDH Modern MXphobicmdNo ratings yet
- Background: Go ToDocument5 pagesBackground: Go ToАлександр КравченкоNo ratings yet
- DD AppDocument3 pagesDD AppVicky XieNo ratings yet
- Successful Treatment of A 14-Year-Old Patient With Intestinal Malrotation With Laparoscopic Ladd Procedure: Case Report and Literature ReviewDocument5 pagesSuccessful Treatment of A 14-Year-Old Patient With Intestinal Malrotation With Laparoscopic Ladd Procedure: Case Report and Literature ReviewHendry JohannesNo ratings yet
- Intestinal Obstruction Due To An Obturator Hernia: A Case Report With A Review of The LiteratureDocument2 pagesIntestinal Obstruction Due To An Obturator Hernia: A Case Report With A Review of The Literaturemudasir61No ratings yet
- Tarlac State UniversityDocument11 pagesTarlac State UniversityJenica DancilNo ratings yet
- MDCT Evaluation of Foreign Bodies and Liquid Aspiration Pneumonia in AdultsDocument9 pagesMDCT Evaluation of Foreign Bodies and Liquid Aspiration Pneumonia in AdultsHafidz ZuhairNo ratings yet
- Student Autopsy Report: Sample: Summary of Clinical HistoryDocument3 pagesStudent Autopsy Report: Sample: Summary of Clinical HistoryGustavo Fernandez DalenNo ratings yet
- Perforated Appendicitis Signs and ImagingDocument17 pagesPerforated Appendicitis Signs and ImagingEloisamay Barrameda EspirituNo ratings yet
- Case ReportDocument3 pagesCase Reportyena hillNo ratings yet
- Pneumo PeritoneumDocument35 pagesPneumo PeritoneumDepinNo ratings yet
- Appendicitis: Mark W. Jones Hassam Zulfiqar Jeffrey G. DeppenDocument7 pagesAppendicitis: Mark W. Jones Hassam Zulfiqar Jeffrey G. DeppenAndi HairunNo ratings yet
- Acute Appendicitis in Adults - Clinical Manifestations and Differential DiagnosisDocument17 pagesAcute Appendicitis in Adults - Clinical Manifestations and Differential Diagnosisnicholas Rojas SIlvaNo ratings yet
- Art 3A10.1186 2F1476 7120 1 2 PDFDocument6 pagesArt 3A10.1186 2F1476 7120 1 2 PDFFachrul AqNo ratings yet
- Congenital Diaphragmatic Hernia in The Neonate - UpToDateDocument36 pagesCongenital Diaphragmatic Hernia in The Neonate - UpToDatewisdom loverNo ratings yet
- Spider AngiomaDocument5 pagesSpider AngiomaChrizzna HaryantoNo ratings yet
- Traumatic Diaphragmatic Hernia Case ReportDocument3 pagesTraumatic Diaphragmatic Hernia Case ReportRangga Duo RamadanNo ratings yet
- Congenital Lobar EmphysemaDocument4 pagesCongenital Lobar EmphysemaIjahss JournalNo ratings yet
- Acute Appendicitis in Adults Clinical Ma PDFDocument27 pagesAcute Appendicitis in Adults Clinical Ma PDFAntonio Zumaque CarrascalNo ratings yet
- Caso Clinico Sp3Document4 pagesCaso Clinico Sp3eduardoNo ratings yet
- Acute Appendicitis of The Appendiceal StumpDocument3 pagesAcute Appendicitis of The Appendiceal StumpXavier JarrínNo ratings yet
- Acute Appendicitis in Adults 202302Document13 pagesAcute Appendicitis in Adults 202302laura correaNo ratings yet
- Diseases of the Liver and Biliary TreeFrom EverandDiseases of the Liver and Biliary TreeAnnarosa FloreaniNo ratings yet
- Burn - Dr. IshandonoDocument16 pagesBurn - Dr. IshandonoRangga Duo RamadanNo ratings yet
- Linkage Dan Pemetaan GenDocument10 pagesLinkage Dan Pemetaan GenRangga Duo RamadanNo ratings yet
- CDH ReportDocument18 pagesCDH ReportRangga Duo RamadanNo ratings yet
- Population (DR Suryadi)Document32 pagesPopulation (DR Suryadi)Rangga Duo RamadanNo ratings yet
- Molecular Genetics of Some Common Mendelian DisordersDocument30 pagesMolecular Genetics of Some Common Mendelian DisordersErwin Charisma PasangNo ratings yet
- Gene DR HartonoDocument6 pagesGene DR HartonoTri BasukiNo ratings yet
- SepsisAlgorithm Deidentified (1) - June 2014 PDFDocument8 pagesSepsisAlgorithm Deidentified (1) - June 2014 PDFRangga Duo RamadanNo ratings yet
- 1 PBDocument4 pages1 PBRangga Duo RamadanNo ratings yet
- 209 11Document3 pages209 11Rangga Duo RamadanNo ratings yet
- Fetal Surgery For Congenital Diaphragmatic Hernia Is Back From Never GoneDocument12 pagesFetal Surgery For Congenital Diaphragmatic Hernia Is Back From Never GoneRangga Duo RamadanNo ratings yet
- Hernia DiafragmaDocument7 pagesHernia Diafragmasentaro2100% (1)
- 820 FullDocument10 pages820 FullRangga Duo RamadanNo ratings yet
- CDH OverviewDocument9 pagesCDH OverviewRangga Duo RamadanNo ratings yet
- Traumatic Diaphragmatic Hernia Case ReportDocument3 pagesTraumatic Diaphragmatic Hernia Case ReportRangga Duo RamadanNo ratings yet
- 01 Maheetha ProgeriaDocument15 pages01 Maheetha ProgeriaRangga Duo RamadanNo ratings yet
- 2014 9056Document12 pages2014 9056Rangga Duo RamadanNo ratings yet
- Kerosene PDFDocument31 pagesKerosene PDFRudyMLanaNo ratings yet
- Assessment Antenatal Screening Down SyndromeDocument107 pagesAssessment Antenatal Screening Down SyndromeRangga Duo RamadanNo ratings yet
- PRFhandbook 0410Document102 pagesPRFhandbook 0410Rangga Duo RamadanNo ratings yet
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Document25 pagesP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Nurul AmaliaNo ratings yet
- Necrotising Enterocolitis: The State of The Science: Kathleen Gibbs, Jing Lin and Ian R. HolzmanDocument6 pagesNecrotising Enterocolitis: The State of The Science: Kathleen Gibbs, Jing Lin and Ian R. HolzmanRangga Duo RamadanNo ratings yet
- Perks ArticleDocument10 pagesPerks ArticleRangga Duo RamadanNo ratings yet
- D 912 F 505 C 061672685Document7 pagesD 912 F 505 C 061672685Rangga Duo RamadanNo ratings yet
- Nutrition For Patients With Hirschsprung's Disease: Patients That Have Surgery Soon After BirthDocument5 pagesNutrition For Patients With Hirschsprung's Disease: Patients That Have Surgery Soon After BirthRangga Duo RamadanNo ratings yet
- 5 TetanusDocument11 pages5 TetanusRangga Duo RamadanNo ratings yet
- Help Pedsurgeryafrica70Document8 pagesHelp Pedsurgeryafrica70Rangga Duo RamadanNo ratings yet
- 2013 Tadulako Prescrpton AnalyssDocument11 pages2013 Tadulako Prescrpton AnalyssRangga Duo RamadanNo ratings yet
- 2013 Tadulako Prescrpton AnalyssDocument11 pages2013 Tadulako Prescrpton AnalyssRangga Duo RamadanNo ratings yet
- Respiratory Emergencies PDFDocument4 pagesRespiratory Emergencies PDFAidan RajkumarNo ratings yet
- An Assessment of Dietary Habit of Obese Among YoungstersDocument27 pagesAn Assessment of Dietary Habit of Obese Among YoungstersIHTESHAM ULHAQNo ratings yet
- P. D. Shere, Et AlDocument11 pagesP. D. Shere, Et Alemilson sorianoNo ratings yet
- Paul Eugen Bleuler and The Origin of The Term Schizophrenia - Ashok Baugh Yeragani 2012Document3 pagesPaul Eugen Bleuler and The Origin of The Term Schizophrenia - Ashok Baugh Yeragani 2012Yahoska TijerinoNo ratings yet
- Pediatric Advanced Life Support (PALS) - UpToDateDocument57 pagesPediatric Advanced Life Support (PALS) - UpToDateSofíaNo ratings yet
- Riya Parikh Essay 4 1Document6 pagesRiya Parikh Essay 4 1api-3026862950% (1)
- Tobacco Reasearch PaperDocument7 pagesTobacco Reasearch Paperapi-316040758No ratings yet
- Sas 2 HaDocument1 pageSas 2 HaKristinelou Marie N. ReynaNo ratings yet
- Research PaperDocument5 pagesResearch PaperAnnie OmlangNo ratings yet
- Biosafety in Microbiological and Biomedical Laboratories: 6th EditionDocument604 pagesBiosafety in Microbiological and Biomedical Laboratories: 6th Editionnam7124119No ratings yet
- Medical Terminology 05Document16 pagesMedical Terminology 05Tuaha MasoodNo ratings yet
- Reviewer Nutrimonth QuizbeeDocument47 pagesReviewer Nutrimonth QuizbeeDale Lyko AbionNo ratings yet
- Health Is WealthDocument1 pageHealth Is Wealthriana safrianiNo ratings yet
- Study of Renal Profile in Obese Hypertensive Patients: Nani Kazi DesharDocument16 pagesStudy of Renal Profile in Obese Hypertensive Patients: Nani Kazi DesharBipin SunarNo ratings yet
- Hypertrophic Cardiomyopathy: Joisy Aloor Leonard Shaju Smit Bhaisare Shawn RyneDocument36 pagesHypertrophic Cardiomyopathy: Joisy Aloor Leonard Shaju Smit Bhaisare Shawn RyneJoisy Aloor100% (1)
- From Pro-Inflammatory Molecules To The Brain's Resting-State Connectivity The Future of Clinical Diagnosis of DepressionDocument36 pagesFrom Pro-Inflammatory Molecules To The Brain's Resting-State Connectivity The Future of Clinical Diagnosis of DepressiondgavrileNo ratings yet
- Ocular InfectionsDocument129 pagesOcular Infectionsnorma paulina carcausto lipaNo ratings yet
- World Breastfeeding Week 2011Document20 pagesWorld Breastfeeding Week 2011Annapurna DangetiNo ratings yet
- Pengenalan DiabetesDocument57 pagesPengenalan DiabetesAsma MandaNo ratings yet
- Daftar Pustaka ImunologiDocument2 pagesDaftar Pustaka ImunologiJacklyn FultonNo ratings yet
- Os Correlatos Anatômicos Subjacentes Da Meditação de Longo Prazo Maiores Volumes Hipocampais e Frontais Da Massa CinzentaDocument18 pagesOs Correlatos Anatômicos Subjacentes Da Meditação de Longo Prazo Maiores Volumes Hipocampais e Frontais Da Massa CinzentasistemaNo ratings yet
- BLOOD PRODUCTS AND PLASMA SUBSTITUTESDocument5 pagesBLOOD PRODUCTS AND PLASMA SUBSTITUTESBramantyo NugrahaNo ratings yet
- Vitamin eDocument12 pagesVitamin eapi-287108173No ratings yet