Professional Documents
Culture Documents
11 Chemistry Structure of Atom Test 05 Answer 67ty
11 Chemistry Structure of Atom Test 05 Answer 67ty
Uploaded by
harsh0 ratings0% found this document useful (0 votes)
7 views2 pagesThe document is a set of answers to questions about the quantum mechanical model of the atom. It discusses Heisenberg's Uncertainty Principle, which states that the exact position and momentum of an electron cannot be determined simultaneously. It also discusses de Broglie's relation that every particle in motion is associated with a wavelength. Finally, it provides an example calculation of the uncertainty in velocity of an electron using the uncertainty principle.
Original Description:
m
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document is a set of answers to questions about the quantum mechanical model of the atom. It discusses Heisenberg's Uncertainty Principle, which states that the exact position and momentum of an electron cannot be determined simultaneously. It also discusses de Broglie's relation that every particle in motion is associated with a wavelength. Finally, it provides an example calculation of the uncertainty in velocity of an electron using the uncertainty principle.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views2 pages11 Chemistry Structure of Atom Test 05 Answer 67ty
11 Chemistry Structure of Atom Test 05 Answer 67ty
Uploaded by
harshThe document is a set of answers to questions about the quantum mechanical model of the atom. It discusses Heisenberg's Uncertainty Principle, which states that the exact position and momentum of an electron cannot be determined simultaneously. It also discusses de Broglie's relation that every particle in motion is associated with a wavelength. Finally, it provides an example calculation of the uncertainty in velocity of an electron using the uncertainty principle.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
CBSE TEST PAPER-05
CLASS - XI CHEMISTRY [ANSWERS]
Topic: - Quantum Mechanical Model of the Atom
Ans1:
It states that
It is impossible to determine simultaneously the exact position and exact
momentum (or velocity) of an electron.
Ans2:
Mathematically, it can be given as
x px h
or , x ( mvx ) h
or , x U x h
4 m
Where x is the uncertainty in position and px ( vx ) is the uncertainty in
momentum (or velocity) of the particle.
Ans3:
If the position of the electron is known with high degree of accuracy ( x is small),
then the velocity of the electron will be uncertain ( (Vx ) is large.) .
Ans4:
According to de Broglies relation, = h
mv
i.e.
1
the mass of the car is very
m
large and its wavelength ( ) or wave character is negligible. Therefore, we do not
see a car moving like a wave.
Ans5:
According to de Broglie, every particle in motion is associated with a wavelength
and other wave characteristics. He deduced the relation that wavelength ( ) of a
particle in motion is equal to the Plancks constant (h) divided by the momentum (p)
of the particle.
i.e. =
h
1
=
p mv
Where m is the mass, v is the velocity after particles
Material downloaded from http://myCBSEguide.com and http://onlineteachers.co.in
Portal for CBSE Notes, Test Papers, Sample Papers, Tips and Tricks
Ans6:
Because there is an uncertainty in the velocity of moving electron around the
nucleus (Heisenbergs Uncertainty Principle).
Ans7:
According to uncertainty Principle
h
4
h
or, p =
4x
x.p =
or , p =
6.626 1034 kgm2 s 1
4 3.143 10 10 m)
= 5.27 1026 kgms 1
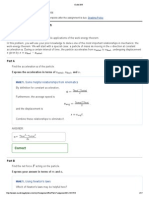
Ans8:
Uncertainty in velocity ( v ) is given by
h
4 mx
6.6 10 34 kgm2 s 1
=
22
4 4 103 kg (10m)
7
= 1.3 10 39 ms 1
v
The uncertainty in the velocity of the wagon is = 1.3 1039 ms 1
Ans9:
2 represent probability of finding an electron.
Material downloaded from http://myCBSEguide.com and http://onlineteachers.co.in
Portal for CBSE Notes, Test Papers, Sample Papers, Tips and Tricks
You might also like
- Quantum Mechanics:Uncertainty PrincipleDocument25 pagesQuantum Mechanics:Uncertainty Principlevivek patelNo ratings yet
- Mastering Physics: CH 06Document27 pagesMastering Physics: CH 06Paulina Wetner75% (24)
- 11th Chemistry Atom Structure-Answer 05Document2 pages11th Chemistry Atom Structure-Answer 05satya176No ratings yet
- Module 4Document25 pagesModule 4Bhavana A100% (1)
- Doc 20230114 Wa0012.Document19 pagesDoc 20230114 Wa0012.mohammedgousmujahidNo ratings yet
- BPHYS102Document19 pagesBPHYS102Prasanna PrasannaNo ratings yet
- Quantum Mechanics - Study NotesDocument18 pagesQuantum Mechanics - Study NotesmadjerkmemesNo ratings yet
- HW 07 202H SolutionsDocument6 pagesHW 07 202H SolutionsronaldhaiatNo ratings yet
- Module 2 Quantum MechanicsDocument64 pagesModule 2 Quantum MechanicsAkshat GuptaNo ratings yet
- LS U1 QuantumDocument21 pagesLS U1 QuantumnamratamanglaniNo ratings yet
- NP Notes - Module 4 Only Quantum MechanicsDocument17 pagesNP Notes - Module 4 Only Quantum MechanicsNightHawkNo ratings yet
- A I Ems Quantum MechaDocument11 pagesA I Ems Quantum MechaRobinson XMINo ratings yet
- Class: Subject: Unit No. & Name: Chapter No. & Name: TopicDocument9 pagesClass: Subject: Unit No. & Name: Chapter No. & Name: TopicMayank ChoudharyNo ratings yet
- PHY 122 WK 21-07-2021 ADocument19 pagesPHY 122 WK 21-07-2021 AgideonNo ratings yet
- BPHYE102 Mod1@AzDOCUMENTS - inDocument14 pagesBPHYE102 Mod1@AzDOCUMENTS - inshreelakshmicbadigerNo ratings yet
- Engg. Physics - II Unit-I: (Wave Mechanics and X-Rays)Document15 pagesEngg. Physics - II Unit-I: (Wave Mechanics and X-Rays)muhammadfaisal32717No ratings yet
- Phy Unit 3 QuantumMechanics PDFDocument18 pagesPhy Unit 3 QuantumMechanics PDFJayanth GuntupalliNo ratings yet
- Module-2, Modern Physics: Black Body RadiationDocument16 pagesModule-2, Modern Physics: Black Body RadiationRohithNo ratings yet
- Unit-I, First ChapterDocument27 pagesUnit-I, First ChapterkalsidipenNo ratings yet
- Physics Assignment PDFDocument15 pagesPhysics Assignment PDFHarsh MittalNo ratings yet
- Quantum 2Document20 pagesQuantum 2dishant sarangalNo ratings yet
- Unit III Quantum MechanicsDocument9 pagesUnit III Quantum MechanicsVoxy. exNo ratings yet
- Quantum Mechanics Contents: Introduction, Heisenberg's Uncertainty Principle - Statement, SignificanceDocument12 pagesQuantum Mechanics Contents: Introduction, Heisenberg's Uncertainty Principle - Statement, Significancepink dollNo ratings yet
- Mod4MOD (Fall2011)Document8 pagesMod4MOD (Fall2011)Muhtasim Ayaz RahmanNo ratings yet
- Mod Ch3matDocument15 pagesMod Ch3matJulian David Henao EscobarNo ratings yet
- Module 1 AY 2022-23Document11 pagesModule 1 AY 2022-23mounikarechNo ratings yet
- Quantum MechanicsDocument10 pagesQuantum MechanicsMahesh Lohith K.SNo ratings yet
- Heisenberg Uncertainty Principle QuestionsDocument6 pagesHeisenberg Uncertainty Principle QuestionsPrem MehrotraNo ratings yet
- De BrouglieDocument6 pagesDe BrouglieJiezel CastorNo ratings yet
- BIT Sindri Chemistry Module 1Document5 pagesBIT Sindri Chemistry Module 1PalNo ratings yet
- Phy1 4Document5 pagesPhy1 4sakshamsharma7257No ratings yet
- SCP Module 1Document12 pagesSCP Module 1ReddyNo ratings yet
- 031 048 PDFDocument18 pages031 048 PDFRaj SaumyaNo ratings yet
- Module 2 - Quantum Mechanics-SNDocument21 pagesModule 2 - Quantum Mechanics-SNrakshithatanNo ratings yet
- Quantum Physics Unit-2ndDocument18 pagesQuantum Physics Unit-2ndMSONI97No ratings yet
- Quantum Mechanics 1Document88 pagesQuantum Mechanics 1Mehul GuptaNo ratings yet
- On The Relativistic Classical Motion of A Radiating Spinning Particle in A Magnetic FieldDocument17 pagesOn The Relativistic Classical Motion of A Radiating Spinning Particle in A Magnetic FieldkichumalluNo ratings yet
- What Are Free Particles in Quantum MechanicsDocument21 pagesWhat Are Free Particles in Quantum MechanicskalshinokovNo ratings yet
- Unit IV Quantum MechanicsDocument15 pagesUnit IV Quantum MechanicsYash100% (1)
- Engineering PhysicsDocument131 pagesEngineering PhysicsG46Anand P KNo ratings yet
- Mit8 323 s23 Rec01Document8 pagesMit8 323 s23 Rec01Origami TutorialsNo ratings yet
- Quantum MechanicsDocument6 pagesQuantum MechanicsRINA MORENONo ratings yet
- Deriving Time Dependent & Independent Schrodinger Equations, Classical and Hamilton Jacobi EquationsDocument18 pagesDeriving Time Dependent & Independent Schrodinger Equations, Classical and Hamilton Jacobi EquationsAumair MalikNo ratings yet
- Uncertainty Principle.: S1 Module I-Notes Part IIDocument7 pagesUncertainty Principle.: S1 Module I-Notes Part IIweak manNo ratings yet
- Homework Set4 MatterwavesDocument2 pagesHomework Set4 MatterwavesyobroskyNo ratings yet
- Part 1Document5 pagesPart 1pelegmeir4No ratings yet
- Phy 206 Rlec 4Document9 pagesPhy 206 Rlec 4ph23b001No ratings yet
- Assignment On Wave Function and Born's Interpretation On Wave FunctionDocument6 pagesAssignment On Wave Function and Born's Interpretation On Wave FunctionMehak MughalNo ratings yet
- Tut-sheet-2-PHL120-13 With Final Answers PDFDocument2 pagesTut-sheet-2-PHL120-13 With Final Answers PDFjgrgpt33No ratings yet
- Quantum Mechanics - 2Document14 pagesQuantum Mechanics - 2Deeptonabho DuttaNo ratings yet
- 2nd Year Chapter 7Document9 pages2nd Year Chapter 7joy bakshiNo ratings yet
- Unit 2 - Quantum MechanicsDocument34 pagesUnit 2 - Quantum MechanicsShivaranjanNo ratings yet
- The Origin of The Algebra of Quantum Operators in The Stochastic Formulation of Quantum Mechanics Mark DavidsonDocument9 pagesThe Origin of The Algebra of Quantum Operators in The Stochastic Formulation of Quantum Mechanics Mark DavidsonflyredeagleNo ratings yet
- Ambjorn Science No30Document23 pagesAmbjorn Science No303mce5eliaNo ratings yet
- QuantumDocument388 pagesQuantumyana33No ratings yet
- Iqbal2020 Article DynamicalInterferenceOfShannonDocument8 pagesIqbal2020 Article DynamicalInterferenceOfShannonhellhawk123No ratings yet
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenFrom EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillNo ratings yet
- ज्यमिति PDFDocument6 pagesज्यमिति PDFharshNo ratings yet
- Set 2 MathDocument12 pagesSet 2 MathharshNo ratings yet
- ChemistryDocument78 pagesChemistryharshNo ratings yet
- Classification of Elements and Periodicity in PropertiesDocument13 pagesClassification of Elements and Periodicity in PropertiesharshNo ratings yet
- WBC PDFDocument1 pageWBC PDFharshNo ratings yet
- XSBJNCDF JK Kybcdm Buicd Bcdujbdklfctdyjx - Lvuyjc D Vtfyuhkjdc SLM Mecdnsxk CDXB b78vb TCVNM Dyv Dyhu DDKV J Ty FBVTGHBV FVSVFDocument1 pageXSBJNCDF JK Kybcdm Buicd Bcdujbdklfctdyjx - Lvuyjc D Vtfyuhkjdc SLM Mecdnsxk CDXB b78vb TCVNM Dyv Dyhu DDKV J Ty FBVTGHBV FVSVFharshNo ratings yet
- World Become Faster WBCDocument1 pageWorld Become Faster WBCharshNo ratings yet