Professional Documents
Culture Documents
Carbene-Stabilized Diarsenic - Diarsene Dications and Diarsenic Radical Cations
Uploaded by
Crystal YoungOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Carbene-Stabilized Diarsenic - Diarsene Dications and Diarsenic Radical Cations
Uploaded by
Crystal YoungCopyright:
Available Formats
Communication
pubs.acs.org/JACS
Oxidation of Carbene-Stabilized Diarsenic: Diarsene Dications and
Diarsenic Radical Cations
Mariham Y. Abraham, Yuzhong Wang, Yaoming Xie, Robert J. Gilliard, Jr., Pingrong Wei, Brian J. Vaccaro,
Michael K. Johnson, Henry F. Schaefer, III, Paul v. R. Schleyer, and Gregory H. Robinson*
Department of Chemistry and the Center for Computational Chemistry, The University of Georgia, Athens, Georgia 30602-2556,
United States
S Supporting Information
*
centered radicals have been studied experimentally only by gasphase electron diraction25 and EPR spectroscopy.20,26
Although gallium halides reportedly are poor oxidizing
reagents,27 Bertrands report of the one-electron oxidation of
the cyclic (alkyl)(amino)carbene (CAAC)-stabilized parent
borylene to the corresponding borinylium complex (HB+)
using GaCl3 is signicant.28 In our laboratory, the reaction of 1
with GaCl3 in a 1:4 ratio in toluene quantitatively resulted in
the orange dicationic diarsene complex 22+[GaCl4]2 (Scheme
1). However, the reaction of 1 with GaCl3 in a 1:2 ratio in Et2O
ABSTRACT: Oxidation of carbene-stabilized diarsenic,
L:AsAs:L [L: = :C{N(2,6-iPr2C6H3)CH}2] (1), with
gallium chloride in a 1:4 ratio in toluene aords the
dicationic diarsene complex [L:AsAs:L]2+([GaCl4])2
(22+[GaCl4]2), while oxidation of 1 with GaCl3 in a 1:2
ratio in Et2O yields the monocationic diarsenic radical
complex [L:AsAs:L]+[GaCl4] (2+[GaCl4]). Strikingly,
complex 2+ is the rst arsenic radical to be structurally
characterized in the solid state. The nature of the bonding
in these complexes was probed computationally and
spectroscopically.
Scheme 1. Gallium Chloride Oxidation of L:AsAs:L
arbene-stabilized diatomic main-group allotropes15 are
intriguing, as they provide convenient zero-oxidationstate platforms from which further chemistry may be explored.
While the rst such complex, carbene-stabilized disilicon,
L:SiSi:L [L: = :C{N(2,6-iPr2C6H3)CH}2], was reported by
this laboratory in 2008,6 subsequent examples of carbenestabilized diatomic main-group allotropes include P2,7,8 As2,9
Ge2,10 B2,11 and Sn2.12 The emerging chemical reactivity of
these molecules is as fascinating as it is provocative. For
example, L:SiSi:L was utilized in the synthesis of a push
pull-stabilized derivative of the parent silylene (:SiH2)13 while
L:PP:L functioned as a bidentate ligand in chelating the BH2+
cation.14 Might carbene-stabilized diarsenic, L:AsAs:L (1),9
similarly possess novel reactivity? Herein we report the
syntheses,15 molecular structures,15 computational studies,16
and electron paramagnetic resonance (EPR) spectra of [L:As
As:L]2+([GaCl4])2 (22+[GaCl4]2) and [L:AsAs:L]+[GaCl4]
(2+[GaCl4]). Complex 22+, a carbene-stabilized diarsene
dication, and complex 2+, a carbene-stabilized monocationic
diarsenic radical cation, were both prepared by oxidation of 1
using gallium chloride. Notably, complex 2+ is the rst stable
arsenic radical to be structurally characterized.
In contrast to transition metals, the main-group p-block
elements do not readily undergo one-electron redox
reactions.17 Regarding the heavier group 15 elements,
persistent18 and stable18 radicals have been experimentally
realized only for phosphorus.1924 Bertrand recently reported
that carbeneP 2 complexes could be oxidized to the
corresponding monocationic P2+ radicals and P22+ dications
using Ph3C+B(C6F5)4 and ferrocenium triate, respectively.19
In contrast, the radical chemistry of arsenic, antimony, and
bismuth is largely unexplored.18 Indeed, persistent arsenic 2013 American Chemical Society
aorded the green monocationic diarsenic radical complex
2+[GaCl4] in 29.1% yield (Scheme 1). Similarly, AlCl3 and
InCl3 were also shown to oxidize 1 to 22+[ECl4]2 (E = Al, In).
Our investigation suggested that the [ECl4] (E = Al, Ga, In)
counteranions may aect the stability of the dicationic [L:As
As:L]2+ fragment in polar solvents. In acetonitrile, while the
orange compounds 22+[ECl4]2 (E = Al, Ga) were stable, orange
22+[InCl4]2 gradually decomposed with the color changing to
green.
The compound 2+[GaCl4] was isolated from the hexane/
THF mixed solvent as dark-green crystals. X-ray structural
analysis15 (Figure 1) showed that the As2 core of 2+ is
disordered (only one set of disordered structural data is shown
in Figure 1) with an average AsAs bond distance of 2.32 ,
which falls between the AsAs single bond distance in 1
[2.442(1) ] and the AsAs double bond distance [2.224(2)
] in RAsAsR [R = 2,4,6-tBu3C6H2; R = CH(SiMe3)2].29
The average AsC bond distances of 1.92 1.95 in 2+ are
somewhat longer than those in 1 [1.881(2) ].9 The computed
Received: January 8, 2013
Published: January 30, 2013
2486
dx.doi.org/10.1021/ja400219d | J. Am. Chem. Soc. 2013, 135, 24862488
Journal of the American Chemical Society
Communication
Figure 1. Molecular structure of 2+. Thermal ellipsoids represent 30%
probability. H atoms have been omitted for clarity. Selected bond
distances () and angles (deg): As(1)As(2) 2.332(3), As(1)C(1)
1.960(4), As(2)C(28) 1.938(5), C(1)N(1) 1.358(5), C(1)N(2)
1.346(5), C(28)N(3) 1.357(5), C(28)N(4) 1.359(5); C(1)
As(1)As(2) 92.10(14), C(28)As(2)As(1) 101.02(16), N(1)
C(1)As(1) 123.9(3), N(2)C(1)As(1) 129.0(3), N(3)C(28)
As(2) 135.3(3), N(4)C(28)As(2) 118.9(3).
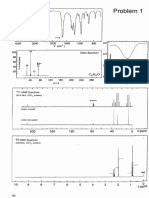
Figure 3. Room-temperature X-band EPR spectrum of 2+ in
uorobenzene (red line) recorded at 9.59 GHz with a modulation
amplitude of 1 mT and a microwave power of 80 mW. Also shown is
the almost perfectly superposed simulated spectrum (black line),
which was generated with the EasySpin software package using a
correlation time of 105 s; gx,y,z = 2.0458, 2.0457, and 2.0452; and two
identical 75As nuclei (I = 3/2) with Ax,y,z = 67.1, 68.0, and 67.8 MHz.
AsAs bond distance of 2.388 in the simplied model 2H+
[in which L: was replaced by :C(NHCH)2; optimized in C2
symmetry]16 is 0.06 longer than that in 2+. The As2-based
localized molecular orbitals (LMOs) of the simplied 2H+
model (Figure 2) include one AsAs bonding orbital (a), one
Figure 4. Molecular structure of 22+. Thermal ellipsoids represent 30%
probability. H atoms have been omitted for clarity. Selected bond
distances () and angles (deg): As(1)As(1A) 2.2803(5), As(1)
C(1) 1.977(2), C(1)N(1) 1.353(3), C(1)N(2) 1.351(3); C(1)
As(1)As(1A) 100.10(7), N(1)C(1)As(1) 135.61(17), N(2)
C(1)As(1) 118.70(17).
Figure 2. LMOs of 2H+ with C2 symmetry: (a) AsAs bonding
orbital; (b) one of the two As lone-pair orbitals; (c) AsAs bonding
orbital; (d) AsAs * antibonding SOMO.
lone-pair orbital on each As atom (b), one AsAs bonding
orbital (c), and one AsAs * singly occupied molecular orbital
(SOMO) (d), which is in accordance with the 1.218 AsAs
Wiberg bond index (WBI). Natural bond orbital (NBO)
analysis30 of 2H+ showed that the As2 core bears a positive
charge of +0.18, which compares to the charge of +0.16 on the
P2 unit in carbene-stabilized P2+ radical (3).19 Similar to 3, the
spin density distribution of 2H+ indicates that the unpaired
electron is largely localized at the As2 core (0.41 at each As
atom), consistent with the SOMO depicted in Figure 2.
The room-temperature EPR spectrum of 2+ in uorobenzene displays a broadened septet (g 2.05) with poorly
resolved low- and high-eld hyperne components resulting
from large hyperne coupling with two equivalent 75As (I =
3
/2) nuclei (A 68 MHz) (Figure 3). Using the correlation
time estimated from parallel NMR studies (105 s), the
spectrum was well-simulated as that of a diarsenic radical
involving equivalent As atoms (the simulation parameters are
shown in the Figure 3 caption). Thus, the EPR data
unambiguously support the radical nature of 2+.
The structural metrics of the present carbene-stabilized
diarsene cations are particularly signicant. Both 22+ (Figure 4)
and 1 possess Ci symmetry, and they exhibit similar trans-bent
geometries with C(1)As(1)As(1A)C(1A) torsion angles
of 180.0 and C(1)As(1)As(1A) bond angles of 100.9,15
The AsAs bond distance in 22+ [2.2803(5) ] is 0.16
shorter than that in 1 [2.442(1) ] and only marginally shorter
than that observed for 2+ (2.32 av). However, the AsAs
bond in 22+ is 0.06 longer than the AsAs double bond
reported for RAsAsR [2.224(2)].29
The AsC bond distances in 22+ [1.977(2) ] are slightly
longer than those in 2+ (1.921.95 av) but 0.1 longer than
those in 1 [1.881(2) ]. The same trend has also been
observed for the changes in the PP and PC bond distances
in carbene-stabilized P27 and its cationic derivatives P2+ and
P22+.19 The computed AsAs (2.296 ) and AsC (1.979 )
bond distances in the simplied model 2H2+ [in which L: was
replaced by :C(NHCH)2]16 are very similar to those in 22+
[AsAs, 2.2803(5) ; AsC, 1.977(2) ]. The LMOs of 2H2+
(Figure 5) indicate the presence of one AsAs bond (a) and
one AsAs bond (b) in 22+. The WBI of 1.78 for 2H2+ is
supportive of arsenicarsenic double-bond character in 22+.
2487
dx.doi.org/10.1021/ja400219d | J. Am. Chem. Soc. 2013, 135, 24862488
Journal of the American Chemical Society
Communication
(13) Abraham, M. Y.; Wang, Y.; Xie, Y.; Wei, P.; Schaefer, H. F., III;
Schleyer, P. v. R.; Robinson, G. H. J. Am. Chem. Soc. 2011, 133, 8874
8876.
(14) Wang, Y.; Xie, Y.; Abraham, M. Y.; Wei, P.; Schaefer, H. F., III;
Schleyer, P. v. R.; Robinson, G. H. Chem. Commun. 2011, 47, 9224
9226.
(15) See the Supporting Information for synthetic and crystallographic details.
(16) The structures of 2H+ and 2H2+ were optimized by density
functional theory at the B3LYP/6-311G** level using the Gaussian 94
and Gaussian 03 programs: (a) Frisch, M. J.; et al. Gaussian 94,
revision B.3; Gaussian, Inc.: Pittsburgh, PA, 1995. (b) Frisch, M. J.;
et al. Gaussian 03, revision C.02; Gaussian, Inc.: Wallingford, CT,
2004.
(17) Power, P. P. Chem. Rev. 2003, 103, 789809.
(18) Stable Radicals: Fundamentals and Applied Aspects of OddElectron Compounds; Hicks, R. G., Ed.; Wiley: Chichester, U.K., 2010;
Chapter 10.
(19) Back, O.; Donnadieu, B.; Parameswaran, P.; Frenking, G.;
Bertrand, G. Nat. Chem. 2010, 2, 369373.
(20) Gynane, M. J. S.; Hudson, A.; Lappert, M. F.; Power, P. P.;
Goldwhite, H. J. Chem. Soc., Chem. Commun. 1976, 623624.
(21) Agarwal, P.; Piro, N. A.; Meyer, K.; Mueller, P.; Cummins, C. C.
Angew. Chem., Int. Ed. 2007, 46, 31113114.
(22) Ishida, S.; Hirakawa, F.; Iwamoto, T. J. Am. Chem. Soc. 2011,
133, 1296812971.
(23) Back, O.; Celik, M. A.; Frenking, G.; Melaimi, M.; Donnadieu,
B.; Bertrand, G. J. Am. Chem. Soc. 2010, 132, 1026210263.
(24) Sasamori, T.; Mieda, E.; Nagahora, N.; Sato, K.; Shiomi, D.;
Takui, T.; Hosoi, Y.; Furukawa, Y.; Takagi, N.; Nagase, S.; Tokitoh, N.
J. Am. Chem. Soc. 2006, 128, 1258212588.
(25) Hinchley, S. L.; Morrison, C. A.; Rankin, D. W. H.; Macdonald,
C. L. B.; Wiacek, R. J.; Voigt, A.; Cowley, A. H.; Lappert, M. F.;
Gundersen, G.; Clyburne, J. A. C.; Power, P. P. J. Am. Chem. Soc. 2001,
123, 90459053.
(26) Gynane, M. J. S.; Hudson, A.; Lappert, M. F.; Power, P. P.;
Goldwhite, H. J. Chem. Soc., Dalton Trans. 1980, 24282433.
(27) Wulfsberg, G. Principles of Descriptive Inorganic Chemistry;
Brooks/Cole: Monterey, CA, 1987.
(28) Kinjo, R.; Donnadieu, B.; Celik, M. A.; Frenking, G.; Bertrand,
G. Science 2011, 333, 610613.
(29) Cowley, A. H.; Lasch, J. G.; Norman, N. C.; Pakulski, M. J. Am.
Chem. Soc. 1983, 105, 55065507.
(30) Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88,
899926.
Figure 5. LMOs of 2H2+ with Ci symmetry: (a) AsAs bonding
orbital; (b) AsAs bonding orbital; (c) one of the AsC bonding
orbitals; (d) one of the As lone-pair orbitals.
The positive partial charge of +0.77 on the As2 core in 22+
compares well with the charge of +0.73 on the P2 unit in
dicationic 3.19
In summary, one- and two-electron oxidations of carbenestabilized diarsenic using gallium chloride were achieved.
Complex 2+ is the rst stable arsenic radical to be structurally
characterized.
ASSOCIATED CONTENT
S Supporting Information
*
Complete refs 16a and 16b and full details of the syntheses,
computations, and X-ray crystal structure determination,
including CIFs. This material is available free of charge via
the Internet at http://pubs.acs.org.
AUTHOR INFORMATION
Corresponding Author
robinson@uga.edu
Notes
The authors declare no competing nancial interest.
ACKNOWLEDGMENTS
We are grateful to the National Science Foundation for
support: CHE-0953484 (G.H.R., Y.W.), CHE-1057466
(P.v.R.S.), and CHE-1054286 (H.F.S.).
REFERENCES
(1) Wang, Y.; Robinson, G. H. Chem. Commun. 2009, 52015213.
(2) Wang, Y.; Robinson, G. H. Inorg. Chem. 2011, 50, 1232612337.
(3) Wang, Y.; Robinson, G. H. Dalton Trans. 2012, 41, 337345.
(4) Martin, D.; Melaimi, M.; Soleilhavoup, M.; Bertrand, G.
Organometallics 2011, 30, 53045313.
(5) Martin, D.; Soleilhavoup, M.; Bertrand, G. Chem. Sci. 2011, 2,
389399.
(6) Wang, Y.; Xie, Y.; Wei, P.; King, R. B.; Schaefer, H. F., III;
Schleyer, P. v. R.; Robinson, G. H. Science 2008, 321, 10691071.
(7) Wang, Y.; Xie, Y.; Wei, P.; King, R. B.; Schaefer, H. F., III;
Schleyer, P. v. R.; Robinson, G. H. J. Am. Chem. Soc. 2008, 130,
1497014971.
(8) Back, O.; Kuchenbeiser, G.; Donnadieu, B.; Bertrand, G. Angew.
Chem., Int. Ed. 2009, 48, 55305533.
(9) Abraham, M. Y.; Wang, Y.; Xie, Y.; Wei, P.; Schaefer, H. F., III;
Schleyer, P. v. R.; Robinson, G. H. Chem.Eur. J. 2010, 16, 432435.
(10) Sidiropoulos, A.; Jones, C.; Stasch, A.; Klein, S.; Frenking, G.
Angew. Chem., Int. Ed. 2009, 48, 97019704.
(11) Braunschweig, H.; Dewhurst, R. D.; Hammond, K.; Mies, J.;
Radacki, K.; Vargas, A. Science 2012, 336, 14201422.
(12) Jones, C.; Sidiropoulos, A.; Holzmann, N.; Frenking, G.; Stasch,
A. Chem. Commun. 2012, 48, 98559857.
2488
dx.doi.org/10.1021/ja400219d | J. Am. Chem. Soc. 2013, 135, 24862488
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Schwedtmann Et Al-2015-Angewandte Chemie International EditionDocument5 pagesSchwedtmann Et Al-2015-Angewandte Chemie International EditionCrystal YoungNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Reflections and MirrorsDocument3 pagesReflections and MirrorsCrystal YoungNo ratings yet
- Capacitance LabDocument6 pagesCapacitance LabCrystal YoungNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Post Lab Data Analysis and QuestionsDocument1 pagePost Lab Data Analysis and QuestionsCrystal YoungNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Knight Ch20Document22 pagesKnight Ch20Crystal YoungNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- 01a MOLECULAR+ORBITALDocument73 pages01a MOLECULAR+ORBITALNovena RasuhNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- 12.01 Characterization of Organometallic ComplexesDocument3 pages12.01 Characterization of Organometallic ComplexesAbeera TehreemNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Huckel TheoryDocument14 pagesHuckel TheoryJahnaviNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- FTIR: Introdution and Application To ForensicsDocument23 pagesFTIR: Introdution and Application To ForensicsLJ GicoleNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- 5.uv - Visible SpectrophotometryDocument92 pages5.uv - Visible SpectrophotometryTare ye TesfuNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Bonding in Polyatomic Molecules: TopicsDocument44 pagesBonding in Polyatomic Molecules: TopicsAcikaNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Chemical Symbols and FormulaDocument2 pagesChemical Symbols and FormulaLakshmyNo ratings yet
- Essay Group 8 - NMRDocument10 pagesEssay Group 8 - NMRMiranti SjahriNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- AFSEM™ - SEM Compatibility List: AFM Integration Into SEMDocument6 pagesAFSEM™ - SEM Compatibility List: AFM Integration Into SEMchangiz2220No ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- LED Optical Characteristics (Efficiency, Power, Freq)Document5 pagesLED Optical Characteristics (Efficiency, Power, Freq)Hasnaa MahmoudNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Chemistry 9th Edition Zumdahl Test BankDocument30 pagesChemistry 9th Edition Zumdahl Test Bankwadeperlid9d98k100% (31)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Nuclear Magnetic Resonance (NMR) Spectroscopy - Instrumentation - Microbe NotesDocument2 pagesNuclear Magnetic Resonance (NMR) Spectroscopy - Instrumentation - Microbe Notesjimgitonga100% (2)
- Ib PPT 4 HL PDFDocument55 pagesIb PPT 4 HL PDFzarna nirmal rawalNo ratings yet
- 2019 - 2020 Chapter 2 Atomic Structure SK015Document71 pages2019 - 2020 Chapter 2 Atomic Structure SK015Jia En TanNo ratings yet
- Mechanisms of Metal Ion Transfer Into Room-Temperature Ionic Liquids: The Role of Anion ExchangeDocument8 pagesMechanisms of Metal Ion Transfer Into Room-Temperature Ionic Liquids: The Role of Anion ExchangeAnge QuintriqueoNo ratings yet
- Why Do Semiconductors Have A Band Gap - QuoraDocument2 pagesWhy Do Semiconductors Have A Band Gap - QuoraSrimonta RoyNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Session-09-Periodocity and PracticeDocument43 pagesSession-09-Periodocity and PracticeMojdeh AnbarfamNo ratings yet
- Synthesis and Studies of PANI/Cerium Dioxide Nanocomposites: E. Kumar, P. SelvarajanDocument4 pagesSynthesis and Studies of PANI/Cerium Dioxide Nanocomposites: E. Kumar, P. SelvarajansobisobiNo ratings yet
- Lewis Dot Structures and Molecular Geometries: Dr. WalkerDocument35 pagesLewis Dot Structures and Molecular Geometries: Dr. WalkerChristine Ano-os RoneNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Chapter 2 Lecture Notes - 0Document44 pagesChapter 2 Lecture Notes - 0KirilKocevskiNo ratings yet
- Tugas InstrumenDocument50 pagesTugas InstrumenMuthi KhairunnisaNo ratings yet
- Dye Surfactant InteractionDocument25 pagesDye Surfactant InteractionHirak ChatterjeeNo ratings yet
- Tuneable Diode Laser Absorption Spectroscopy (TDLAS) - Michell Instruments UK, Dew Point, Humidity and Oxygen SpecialistsDocument1 pageTuneable Diode Laser Absorption Spectroscopy (TDLAS) - Michell Instruments UK, Dew Point, Humidity and Oxygen SpecialistsAlledyne RodriguezNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Fluorimetry: Mol Ecul Ar Luminescence AND LuminescenceDocument41 pagesFluorimetry: Mol Ecul Ar Luminescence AND LuminescencePoornima ANNo ratings yet
- Topic 2 - Atomic Structure - Part 2 - AnswersDocument17 pagesTopic 2 - Atomic Structure - Part 2 - Answersnikes 1No ratings yet
- Chapter 11 InorgDocument12 pagesChapter 11 InorgMauritiusFeliciano100% (6)
- Successive Ionization EnergiesDocument2 pagesSuccessive Ionization EnergiesOutward CauseNo ratings yet
- What Is Optical Emission Spectroscopy (OES)Document20 pagesWhat Is Optical Emission Spectroscopy (OES)Sylab InstrumentsNo ratings yet
- CompDocument2 pagesCompGayatri DasNo ratings yet
- CHM02 - CO1 - LESSON2 - Intermolecular ForceDocument20 pagesCHM02 - CO1 - LESSON2 - Intermolecular ForceMeg IglianeNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)