Professional Documents
Culture Documents
1lab 7 Addendum - Table of Activity Coeficients - Spr07
Uploaded by
Anonymous Now8hfOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1lab 7 Addendum - Table of Activity Coeficients - Spr07
Uploaded by
Anonymous Now8hfCopyright:
Available Formats
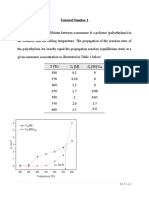
Chem 160 K.
Marr
Addendum to Lab 7.
Activity Coefficients
Table 5.
Thermodynamic Prediction of Precipitation Reactions
(Source: http://www.kayelaby.npl.co.uk/chemistry/3_9/3_9_6.html)
Activity coefficients, , as a function of molality for some strong electrolytes in
aqueous solution at 25 C at different molalities.
molality, m (mol kg1)
Compound
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
Na2SO4
0.446
0.366
0.321
0.291
0.268
0.251
0.236
0.224
0.213
0.204
BaCl2
0.492
0.436
0.412
0.398
0.391
0.388
0.387
0.388
0.390
0.393
CaCl2
0.517
0.469
0.451
0.442
0.444
0.449
0.456
0.467
0.480
0.496
NaNO3
0.762
0.703
0.638
0.599
0.570
0.548
SrCl2
0.510
0.459
0.438
0.428
0.425
0.426
0.430
0.437
0.445
0.456
MgCl2
0.535
0.494
0.481
0.480
0.485
0.496
0.511
0.530
0.552
0.577
MnCl2
0.511
0.464
0.445
0.438
0.436
0.440
0.445
0.453
0.464
0.476
FeCl2
0.509
0.463
0.446
0.441
0.443
0.449
0.458
0.470
0.484
0.500
ZnCl2
0.499
0.447
0.419
0.400
0.384
0.371
0.359
0.348
0.339
0.330
CoCl2
0.529
0.483
0.467
0.463
0.465
0.472
0.482
0.496
0.513
0.532
NiCl2
0.527
0.482
0.466
0.462
0.465
0.473
0.484
0.499
0.517
0.538
CuCl2
0.495
0.441
0.418
0.407
0.407
0.398
0.398
0.399
0.402
0.405
MgBr2
0.540
0.512
0.511
0.522
0.540
0.565
0.595
0.629
0.669
0.715
CaBr2
0.540
0.502
0.491
0.492
0.500
0.513
0.530
0.551
0.576
0.604
SrBr2
0.535
0.492
0.477
0.473
0.476
0.483
0.494
0.508
0.525
0.545
BaBr2
0.512
0.463
0.444
0.437
0.436
0.438
0.443
0.451
0.460
0.471
Mg(NO3)2
0.526
0.484
0.470
0.467
0.471
0.479
0.491
0.508
0.521
0.540
Ca(NO3)2
0.494
0.433
0.402
0.382
0.369
0.359
0.352
0.348
0.344
0.342
Page 1 of 3
Chem 160 K. Marr
Sr(NO3)2
0.482
0.414
0.376
0.350
0.331
0.316
0.304
0.294
0.285
0.278
Co(NO3)2
0.515
0.469
0.452
0.445
0.446
0.450
0.457
0.467
0.478
0.492
Cu(NO3)2
0.516
0.466
0.445
0.435
0.431
0.431
0.434
0.440
0.447
0.456
Li2SO4
0.469
0.400
0.364
0.341
0.325
0.312
0.303
0.296
0.289
0.285
K2SO4
0.424
0.343
0.300
0.272
0.251
0.236
0.224
Rb2SO4
0.443
0.365
0.323
0.295
0.274
0.258
0.245
0.234
0.225
0.217
Cs2SO4
0.444
0.369
0.329
0.304
0.285
0.270
0.259
0.249
0.241
0.234
H2SO4
0.251
0.195
0.171
0.156
0.147
0.140
0.135
0.130
0.127
0.125
When calculating the G of a reaction at nonstandard conditions it is more accurate to use
activities rather than molarity to calculate the reaction quotient, Q.
Activity = (Activity Coefficient)(Molarity):
a = . M
Grxn = Gorxn + RT ln Q
Table 6.
Activity coefficients as a function of molality for some strong monovalent electrolytes
in aqueous solution at 25 C at different molalities
Compound
molality,
m(mol kg1) NaNO3
HCl
LiCl
NaCl
KCl
CsCl
LiNO3
KNO3
CsNO3
0.01
0.900
0.904
0.903
0.902
0.901
0.899
0.903
0.897
0.896
0.02
0.866
0.875
0.873
0.870
0.868
0.865
0.872
0.861
0.860
0.05
0.811
0.830
0.825
0.820
0.816
0.807
0.825
0.799
0.795
0.10
0.762
0.796
0.790
0.778
0.770
0.756
0.788
0.739
0.733
0.2
0.703
0.767
0.757
0.735
0.718
0.694
0.752
0.663
0.655
0.4
0.638
0.755
0.740
0.693
0.666
0.628
0.728
0.576
0.561
0.6
0.599
0.763
0.743
0.673
0.637
0.589
0.727
0.519
0.501
Page 2 of 3
Chem 160 K. Marr
0.8
0.570
0.783
0.755
0.662
0.618
0.563
0.733
0.476
0.458
1.0
0.548
0.809
0.774
0.657
0.604
0.544
0.743
0.443
0.422
1.2
0.530
0.840
0.796
0.654
0.593
0.529
0.757
0.414
0.393
1.4
0.514
0.876
0.823
0.655
0.586
0.518
0.774
0.390
0.368
1.6
0.501
0.916
0.853
0.657
0.580
0.509
0.792
0.369
1.8
0.489
0.960
0.885
0.662
0.576
0.501
0.812
0.350
2.0
0.478
1.009
0.921
0.668
0.573
0.495
0.835
0.333
2.5
0.455
1.147
1.026
0.688
0.569
0.484
0.896
0.297
3.0
0.437
1.316
1.156
0.714
0.569
0.478
0.966
0.269
3.5
0.422
1.518
1.317
0.746
0.572
0.474
1.044
0.246
4.0
0.408
1.762
1.510
0.783
0.577
0.473
1.125
4.5
0.396
2.04
1.741
0.826
0.583
0.473
1.215
5.0
0.386
2.38
2.02
0.874
0.474
1.310
The values of for the activity coefficients, , for the electrolytes in these tables are from Hamer and
Wu (1972). They are principally derived from experimental values of relative water vapour
pressures by use of the GibbsDuhem relation. Values are compiled from NIST (formerly NBS)
tabulations of Goldberg et al. (1977, 1978, 1979a, 1979b, 1981) and the computer compilation
GAMPHI (Goldberg, 1985). Experimental values have also been compiled by Lobo (1989).
Page 3 of 3
You might also like
- 117 Labreport #2Document21 pages117 Labreport #2Glaize Anne Gamelong100% (1)
- Activity CoefficientsDocument14 pagesActivity CoefficientsKenny Then Soon HungNo ratings yet
- Chemistry Answer KeyDocument8 pagesChemistry Answer KeyNnaer Ortiz NasupmilacNo ratings yet
- Durability Study of Low Calcium Fly Ash GeopolymerDocument8 pagesDurability Study of Low Calcium Fly Ash GeopolymerARNABNo ratings yet
- Modeling of Nature and Strength of Acid Centres in U - 1999 - Studies in SurfaceDocument4 pagesModeling of Nature and Strength of Acid Centres in U - 1999 - Studies in SurfaceLindsey BondNo ratings yet
- Iodine Clock Reaction ReportDocument7 pagesIodine Clock Reaction ReportMohamed Shalan0% (1)
- DATOS PARA DIFERENTES MEZCLAS 1butanol Agua Acetona 2 PropanolDocument9 pagesDATOS PARA DIFERENTES MEZCLAS 1butanol Agua Acetona 2 PropanolAndresDiazNo ratings yet
- Cosmosac Regression PresentationDocument27 pagesCosmosac Regression PresentationRashedul IslamNo ratings yet
- Lind S. C., Rosenblum C. - The Combination of Carbon Monoxide and Oxygen Under The Influence of Radon (1932) PDFDocument13 pagesLind S. C., Rosenblum C. - The Combination of Carbon Monoxide and Oxygen Under The Influence of Radon (1932) PDFMiguel MagallanesNo ratings yet
- Communication: Cuo/Sio: A Simple and Efficient Solid Acid Catalyst For Epoxide Ring OpeningDocument4 pagesCommunication: Cuo/Sio: A Simple and Efficient Solid Acid Catalyst For Epoxide Ring Openingfedericasantoro81No ratings yet
- The Enthalpy of Dilution and Apparent Molar Heat Capacity of Naoh (Aq) To 523 K and 40 MpaaDocument24 pagesThe Enthalpy of Dilution and Apparent Molar Heat Capacity of Naoh (Aq) To 523 K and 40 MpaaJaelani AlchotriNo ratings yet
- Improvements and Modeling of The Rwgs ProcessDocument3 pagesImprovements and Modeling of The Rwgs ProcessJonathan WhitlowNo ratings yet
- Assignment 3Document4 pagesAssignment 3Yi Hong LowNo ratings yet
- 1985 The Phase Diagram of The System NaNO-KNO3 Studied by Differential Scanning CalorimetryDocument8 pages1985 The Phase Diagram of The System NaNO-KNO3 Studied by Differential Scanning CalorimetryAdrian CaraballoNo ratings yet
- V O - Smvo Mechanical Mixture: Oxidative Dehydrogenation of PropaneDocument11 pagesV O - Smvo Mechanical Mixture: Oxidative Dehydrogenation of PropaneRebeca CristinaNo ratings yet
- CALPHAD: Computer Coupling of Phase Diagrams and ThermochemistryDocument12 pagesCALPHAD: Computer Coupling of Phase Diagrams and ThermochemistryJarek Jhoel Alejandro ZarateNo ratings yet
- D.1 Barium Klorida (Bacl) : Pelarut ODocument24 pagesD.1 Barium Klorida (Bacl) : Pelarut OSundari PratiwiNo ratings yet
- Experiment 3Document9 pagesExperiment 3Marygrace ProgellaNo ratings yet
- Vapour-Liquid Equilibrium of CO in Aqueous Solutions of 2-Amino-2-Methyl-1-PropanolDocument18 pagesVapour-Liquid Equilibrium of CO in Aqueous Solutions of 2-Amino-2-Methyl-1-PropanolmppatilmayurNo ratings yet
- Lab Report 4Document24 pagesLab Report 4aidoo3045No ratings yet
- CHM1045C Test 2 ReviewDocument3 pagesCHM1045C Test 2 ReviewBryant LuNo ratings yet
- HWK 1Document2 pagesHWK 1Ashwin NairNo ratings yet
- Honors Chem 1st Semester Exam Study Guide Fall 2010Document5 pagesHonors Chem 1st Semester Exam Study Guide Fall 2010Aref DahabrahNo ratings yet
- 1.0 Title of Experiment: BKF2741 Chemical Reaction Engineering Laboratory IDocument4 pages1.0 Title of Experiment: BKF2741 Chemical Reaction Engineering Laboratory IAs Eleyana100% (2)
- Chemistry Lab ReportDocument20 pagesChemistry Lab ReportHermann MurielNo ratings yet
- Cre Lab 9Document3 pagesCre Lab 9Fahad kamranNo ratings yet
- The Conductance of Strong and Weak ElectrolytesDocument8 pagesThe Conductance of Strong and Weak Electrolytessexycassie100% (6)
- 0010 - El-Hammamy 2011Document5 pages0010 - El-Hammamy 2011Amr GamalNo ratings yet
- Enzymes & Biological CatalysisDocument64 pagesEnzymes & Biological Catalysisjoer13No ratings yet
- cg501386j Si 005Document8 pagescg501386j Si 005Mónica Ayala GómezNo ratings yet
- KWInorganic Chem PS 1 PDFDocument1 pageKWInorganic Chem PS 1 PDFmaeNo ratings yet
- Experiment 3 ReportDocument6 pagesExperiment 3 ReportDolly NarisNo ratings yet
- Kinetics Prelim Take Home Exam December 25 2017Document2 pagesKinetics Prelim Take Home Exam December 25 2017Michelle Mendoza100% (1)
- Hoagland Solution ModifiedDocument1 pageHoagland Solution Modifiedalam19713358No ratings yet
- Duque Bernal2012Document9 pagesDuque Bernal2012SergioNo ratings yet
- Thermofluids Property TablesDocument89 pagesThermofluids Property Tablesthejackal205No ratings yet
- Cre S16Document4 pagesCre S16vikas patheNo ratings yet
- Separation of Azeotropic Mixture Ethanol-Heptane by Solvent Extraction With A Ionic LiquidDocument3 pagesSeparation of Azeotropic Mixture Ethanol-Heptane by Solvent Extraction With A Ionic LiquidGirl96GRNo ratings yet
- 2020 - Equilibrium Practice ProblemsDocument5 pages2020 - Equilibrium Practice ProblemsAMOS SODJAHINNo ratings yet
- CH330 GroupC Plug Flow ReactorDocument8 pagesCH330 GroupC Plug Flow Reactorunnati singhNo ratings yet
- Namazi An 2003Document7 pagesNamazi An 2003nabilNo ratings yet
- 201cs191-Kannan Kumar K c2Document11 pages201cs191-Kannan Kumar K c2KANNAN KUMAR KNo ratings yet
- Potentially Useful Information:: Chemistry 118-03 (10:00 Am MWF) Exam 3 Fall 2010 NameDocument6 pagesPotentially Useful Information:: Chemistry 118-03 (10:00 Am MWF) Exam 3 Fall 2010 NamemusicfeverNo ratings yet
- Chapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HallDocument23 pagesChapter 27: Physical Properties Rules of Thumb For Chemical Engineers, 5th Edition by Stephen HalljnmanivannanNo ratings yet
- Group 1 - ALEJANO - DEZOLLER - GRATIS - MIXED FLOW REACTOR-5Document13 pagesGroup 1 - ALEJANO - DEZOLLER - GRATIS - MIXED FLOW REACTOR-5John Frix AlejanoNo ratings yet
- Homework #3Document4 pagesHomework #3isaackotachieNo ratings yet
- Module 07 Batch ReactorDocument4 pagesModule 07 Batch ReactorFarah -HNo ratings yet
- 2017 Past Paper CCSTDocument4 pages2017 Past Paper CCSTShing Fung ChengNo ratings yet
- Qua&fy: Chemistry of Salt-Catalyzed Degradation of Sucrose IN Concentrated Aqueous SolutionsDocument4 pagesQua&fy: Chemistry of Salt-Catalyzed Degradation of Sucrose IN Concentrated Aqueous SolutionsVishnuNo ratings yet
- Materials: Liquid Regions of Lanthanum-Bearing AluminosilicatesDocument16 pagesMaterials: Liquid Regions of Lanthanum-Bearing Aluminosilicatesزھرة ٱلبيلسآنNo ratings yet
- Tutorial Number 1jjkDocument4 pagesTutorial Number 1jjkSaif JassimNo ratings yet
- Conductometry - Overview ChemistryDocument13 pagesConductometry - Overview Chemistryadithya bachhaNo ratings yet
- 7376211SE129 ReportDocument16 pages7376211SE129 ReportGOWTHAM SNo ratings yet
- Chemistry 1A Fall 2010 Exam 2 Key Chapters 4 (Part), 5, 6, and 7 (Part)Document7 pagesChemistry 1A Fall 2010 Exam 2 Key Chapters 4 (Part), 5, 6, and 7 (Part)jasminp8No ratings yet
- Batch ReactorDocument4 pagesBatch ReactorFoo Xiao BingNo ratings yet
- 10 Pb-O - S Phase Stability Diagram at 850.000 C Log Po2 (G)Document2 pages10 Pb-O - S Phase Stability Diagram at 850.000 C Log Po2 (G)Cynthia MerazNo ratings yet
- CH 23Document11 pagesCH 23monogodNo ratings yet
- Chemical, Biochemical, and Engineering Thermodynamics: Stanley I. SandlerDocument5 pagesChemical, Biochemical, and Engineering Thermodynamics: Stanley I. SandlerAriadne ChuaNo ratings yet
- Nanoporous Catalysts for Biomass ConversionFrom EverandNanoporous Catalysts for Biomass ConversionFeng-Shou XiaoNo ratings yet