Professional Documents
Culture Documents
Beaker: Materials and Methods Materials Needed
Uploaded by
Belle0 ratings0% found this document useful (0 votes)
3 views2 pagesThe document outlines the materials and procedure for extracting caffeine from tea leaves. The procedure involves: 1) boiling tea leaves in water to create an aqueous extract; 2) extracting the aqueous solution three times with dichloromethane in a separatory funnel to transfer the caffeine to the organic layer; 3) evaporating the combined organic layers to dryness and weighing the caffeine residue to calculate percent recovery.
Original Description:
org chem
Original Title
fdfnas,dnfknalknsadklf.
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document outlines the materials and procedure for extracting caffeine from tea leaves. The procedure involves: 1) boiling tea leaves in water to create an aqueous extract; 2) extracting the aqueous solution three times with dichloromethane in a separatory funnel to transfer the caffeine to the organic layer; 3) evaporating the combined organic layers to dryness and weighing the caffeine residue to calculate percent recovery.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views2 pagesBeaker: Materials and Methods Materials Needed
Uploaded by
BelleThe document outlines the materials and procedure for extracting caffeine from tea leaves. The procedure involves: 1) boiling tea leaves in water to create an aqueous extract; 2) extracting the aqueous solution three times with dichloromethane in a separatory funnel to transfer the caffeine to the organic layer; 3) evaporating the combined organic layers to dryness and weighing the caffeine residue to calculate percent recovery.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
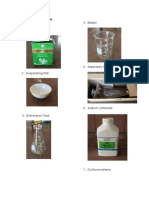
Materials and Methods
Materials Needed:
4. Beaker
1. Tea Bags
5. Separatory Funnel
2. Evaporating Dish
6. Sodium Carbonate
3. Erlenmeyer Flask
7. Dichloromethane
Add 10 g of tea leaves
contained in a tea bag in the
said mixture. Cover the flask
and boil the tea mixture for 10
mins in a low flame.
Remove the liquid and squeeze
the bag. Allow the extract to
cool to room temperature.
Part 2:
8. Sodium Sulfate
Extract the aqueous solution
three times with 20 mL of
dichloromethane each time in a
separatory funnel. Swirl and let
it stand for a few minutes until
until the separation is clearly
visible.
Drain the organic layer in a
clean Erlenmeyer flask. Discard
the aqueous solution. Combine
all the organic layer extracts.
Part 3:
Procedure:
Part 1:
Place 4.4 g of Anhydrous
Sodium Carbonate in a 500 mL
beaker and add 100 mL of
distilled water. Heat in a water
bath until the solid dissolves.
Transfer the extract into an
Erlenmeyer flask containing half
a spatula of anhydrous sodium
sulfate.
Decant into a tared evaporating
dish. Evaporate to dryness
under the hood.
Weigh the residue and calculate
the percentage recovery of
caffeine.
You might also like
- Extraction of Caffeine From TeaDocument2 pagesExtraction of Caffeine From TeaIshan ModiNo ratings yet
- Experiment FiveDocument4 pagesExperiment FiveSusana DakoraNo ratings yet
- Lab Practical ProcedureDocument2 pagesLab Practical ProcedureAlyssa FerenceNo ratings yet
- Caffeine LabDocument3 pagesCaffeine LabSneha KannothNo ratings yet
- Experiment #6 - Isolation of Caffeine From Tea LeavesDocument4 pagesExperiment #6 - Isolation of Caffeine From Tea LeavesAmritRanjanNo ratings yet
- Orange IiDocument6 pagesOrange IiDini KartikaNo ratings yet
- Reagents Required:: Extraction StepDocument4 pagesReagents Required:: Extraction StepZahratulMulazamahNo ratings yet
- DNA BananasDocument9 pagesDNA BananasNigar CəlilovaNo ratings yet
- CHM2210 LAB Caffeine Isolation POST LABDocument3 pagesCHM2210 LAB Caffeine Isolation POST LABgabeverizonNo ratings yet
- Emmanuel Mawere: Experiment ProcedureDocument2 pagesEmmanuel Mawere: Experiment ProcedurekudaNo ratings yet
- Caffein Extraction of Tea LeavesDocument5 pagesCaffein Extraction of Tea LeavesAswin SuginiNo ratings yet
- Preserves Recipes - Prepared or preserved easy: Fast, Easy & Delicious Cookbook, #1From EverandPreserves Recipes - Prepared or preserved easy: Fast, Easy & Delicious Cookbook, #1No ratings yet
- That XXDocument2 pagesThat XXNuriana FajarNo ratings yet
- I. PROSEDUR Dari Orange 2Document4 pagesI. PROSEDUR Dari Orange 2Nuriana FajarNo ratings yet
- Separating The Components of Panacetin PrelabDocument5 pagesSeparating The Components of Panacetin PrelabAmy LaPointe100% (2)
- Extraction of Catteine in Tea LeavesDocument8 pagesExtraction of Catteine in Tea LeavesShanaz ShaxawanNo ratings yet
- Synthesis of Alum From Scrap Aluminum LabDocument2 pagesSynthesis of Alum From Scrap Aluminum Labzack123321No ratings yet
- Lab Report - Preparation of Inorganic CompoundsDocument5 pagesLab Report - Preparation of Inorganic CompoundsDexter ClamohoyNo ratings yet
- Caffeine LabDocument11 pagesCaffeine Labapi-335483695100% (1)
- The Extraction of Caffeine From TeaDocument18 pagesThe Extraction of Caffeine From Teaapi-255504065100% (1)
- Extracting DNA From BananasDocument1 pageExtracting DNA From BananasJennifer Alambra SiñelNo ratings yet
- Alcool Din TarateDocument3 pagesAlcool Din TarateKifa PetraNo ratings yet
- Chemistry Investigatory ProjectDocument14 pagesChemistry Investigatory ProjectChiquita LemosNo ratings yet
- Experiment 3 - Lab Manual - 5f573ea37f406096d489c2ad25 - 230417 - 122117Document3 pagesExperiment 3 - Lab Manual - 5f573ea37f406096d489c2ad25 - 230417 - 122117Retardo Meal O'sNo ratings yet
- PracticalsDocument20 pagesPracticalshariharanNo ratings yet
- 7-Extraction and Recrystallization of Caffeine From Tea (P)Document5 pages7-Extraction and Recrystallization of Caffeine From Tea (P)Gezem GigantoNo ratings yet
- ALENGOSVIG's HowTo Upped by Met A FractalDocument15 pagesALENGOSVIG's HowTo Upped by Met A FractalPhileasNo ratings yet
- Synthesis of Acetylsalicylic AcidDocument14 pagesSynthesis of Acetylsalicylic AcidSampathirao SidharthNo ratings yet
- Activity 10 Extraction and Recrystallization of Caffeine From Tea ProcedureDocument5 pagesActivity 10 Extraction and Recrystallization of Caffeine From Tea Procedurejessie jacolNo ratings yet
- Perfume RecipeDocument2 pagesPerfume RecipeKaye SimbolNo ratings yet
- Experiment 6 - Sem2Document4 pagesExperiment 6 - Sem2MUHAMMAD SYUKRI FITRI BIN MOHAMAD RAZALINo ratings yet
- Multiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneDocument5 pagesMultiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneChristian VillanuevaNo ratings yet
- Science ProjectDocument10 pagesScience Projectmusic pleaseNo ratings yet
- Null 2Document4 pagesNull 2gehanhefina804No ratings yet
- Organic Chemistry Exp.3Document3 pagesOrganic Chemistry Exp.3Aws SarajNo ratings yet
- Tea ProjDocument9 pagesTea Projapi-2000111170% (10)
- Acidity of Tea LeavesDocument17 pagesAcidity of Tea LeavesAviral 1775% (20)
- How To Make Vinegar From Banana PeelingsDocument2 pagesHow To Make Vinegar From Banana PeelingsAnne CervantesNo ratings yet
- Procedure: Experiment: The Effect of Substrate Concentration On Enzyme ActivityDocument2 pagesProcedure: Experiment: The Effect of Substrate Concentration On Enzyme Activitysalahuddin_md5935No ratings yet
- Denaturing ProteinsDocument13 pagesDenaturing ProteinsFerds SalvatierraNo ratings yet
- Tabletop Methamphetamines Uncle FesterDocument3 pagesTabletop Methamphetamines Uncle FesterJonathan MarkNo ratings yet
- Risk AssessmentDocument2 pagesRisk AssessmentfrancescoNo ratings yet
- Caffeine ExtractionDocument2 pagesCaffeine ExtractionUmaidellaNo ratings yet
- 一鍋Document6 pages一鍋METH100% (2)
- Unit 5 - Tong Hop Huu Co-K70Document2 pagesUnit 5 - Tong Hop Huu Co-K70Nguyễn Thu ThủyNo ratings yet
- Isolation of Caffeine From Tea PDFDocument6 pagesIsolation of Caffeine From Tea PDFDaisy Joyce Seroje BuslonNo ratings yet
- Content 2022-23 ExptDocument8 pagesContent 2022-23 ExptvarshNo ratings yet
- The Synthesis of AspirinDocument4 pagesThe Synthesis of AspirinMarieya Elizabeth ZantuaNo ratings yet
- DNA SpoolingDocument3 pagesDNA SpoolingOladejo TaosifatNo ratings yet
- K12 Outreach Solvent Extraction DRAFTDocument8 pagesK12 Outreach Solvent Extraction DRAFTDakota EllisonNo ratings yet
- Chem Final ProjectDocument24 pagesChem Final ProjectAtharva ChaudhariNo ratings yet
- Separation TechniquesDocument4 pagesSeparation TechniquesNicola Faye BronNo ratings yet
- Anaranjado de MetiloDocument2 pagesAnaranjado de MetiloLuis GbNo ratings yet
- Laboratory ExperimentsDocument9 pagesLaboratory ExperimentsSandra MacatangayNo ratings yet
- Brown Veal Stock: ObjectivesDocument2 pagesBrown Veal Stock: ObjectivesMajieNo ratings yet
- Single Solvent RecrystallizationDocument3 pagesSingle Solvent RecrystallizationneesannnNo ratings yet
- Experiment #2: Steam Distillation of Essential Oils Chemistry 102Document5 pagesExperiment #2: Steam Distillation of Essential Oils Chemistry 102Maria LavenderNo ratings yet
- Scalp 2Document1 pageScalp 2BelleNo ratings yet
- Name of Compound Chemica L Formula Uses Picture Barium Chloride Ba CL WaterDocument2 pagesName of Compound Chemica L Formula Uses Picture Barium Chloride Ba CL WaterBelleNo ratings yet
- Name of Compound Chemica L Formula Uses Picture Barium Chloride Ba CL WaterDocument3 pagesName of Compound Chemica L Formula Uses Picture Barium Chloride Ba CL WaterBelleNo ratings yet
- Vitamin C (Ascorbic Acid) A. Oral Brand Name Manufacturer: 1. 2. Alka-C 3. Alka-C CapsuleDocument1 pageVitamin C (Ascorbic Acid) A. Oral Brand Name Manufacturer: 1. 2. Alka-C 3. Alka-C CapsuleBelleNo ratings yet
- Beaker: Materials and Methods Materials NeededDocument2 pagesBeaker: Materials and Methods Materials NeededBelleNo ratings yet
- Beaker: Materials and Methods Materials NeededDocument2 pagesBeaker: Materials and Methods Materials NeededBelleNo ratings yet
- Revised Mod2 With Cover UpdateDocument19 pagesRevised Mod2 With Cover UpdateJerome Rivera CastroNo ratings yet
- Alessandro VoltaDocument3 pagesAlessandro VoltaBelleNo ratings yet
- My Internship ExperienceDocument1 pageMy Internship ExperienceBelleNo ratings yet
- Alessandro VoltaDocument3 pagesAlessandro VoltaBelleNo ratings yet
- Slide 1 - OxygenDocument6 pagesSlide 1 - OxygenBelleNo ratings yet
- Inhalant S 2Document1 pageInhalant S 2BelleNo ratings yet
- Slide 1 - OxygenDocument6 pagesSlide 1 - OxygenBelleNo ratings yet
- ChemDocument1 pageChemBelleNo ratings yet
- Pharchm 1Document1 pagePharchm 1BelleNo ratings yet
- Pharchm 1Document1 pagePharchm 1BelleNo ratings yet