Professional Documents
Culture Documents
Image J Measure OD
Image J Measure OD
Uploaded by
anon_431242846Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Image J Measure OD
Image J Measure OD
Uploaded by
anon_431242846Copyright:
Available Formats
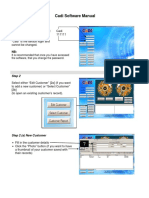
This is what I do for DAB quantification on slides with hematoxylin counterstain:
1. Download the free "Fiji" version of ImageJ from http://fiji.sc. All subsequent steps are
performed in Fiji.
2. Open a DAB image.
3. Run Image > Color > Colour Deconvolution.
4. From the Vectors pull-down, choose "H DAB" as the stain (this assumes your images are
correctly white-balanced, otherwise you have to define your own colors by choosing "From
ROI" for the vector then drawing your own Regions Of Interest to define the stain colors).
5. Click OK in the Colour Deconvolution window, you will get three new images. The one
with "Colour_2" in the title is the DAB image (Colour_1 is the hematoxylin image), you will
quantify the Colour_2 image.
6. Run Analyze > Set Measurements and select "Mean gray value" and "Display label".
7. Select the Colour_2 image window.
8. Run Analyze > Measure (or press Ctrl-m); a "Results" window will pop up with the
quantification in units of intensity.
9. You need to convert the intensity numbers in the Results window to Opitcal Density (OD)
numbers with the following formula:
OD = log(max intensity/Mean intensity), where max intensity = 255 for 8-bit images.
This will quantify the average darkness of the image due to DAB signal. If you have areas of
the image without tissue it will bias your results because it will bring the average OD down.
If that is the case we need to discuss further. Also, you may need to use Integrated Density
rather than Mean.
Good luck and let me know if you need anything else.
You might also like
- Vero Visi Mould TutorialDocument142 pagesVero Visi Mould TutorialAmitava Datta67% (3)
- Hands-On Lab 5 - Different Methods For Creating Dashboard Visualizations With Cognos Analytics (45 Min)Document14 pagesHands-On Lab 5 - Different Methods For Creating Dashboard Visualizations With Cognos Analytics (45 Min)Galacaesar Khambali0% (1)
- Lab 03 - Data Formats - Contrast Stretching - Density SlicingDocument11 pagesLab 03 - Data Formats - Contrast Stretching - Density SlicingANN SHALITANo ratings yet
- VP11 2 Color-LabDocument10 pagesVP11 2 Color-LabinigofetNo ratings yet
- Labklass Digital Image AnalysisDocument6 pagesLabklass Digital Image AnalysiscabrahaoNo ratings yet
- Photoshop TipsDocument45 pagesPhotoshop Tipst1029No ratings yet
- Cell AnalysisDocument4 pagesCell AnalysisPeter HongNo ratings yet
- Colour Analysis Tools in ImagejDocument23 pagesColour Analysis Tools in ImagejPatricio SaucedaNo ratings yet
- Using Imagej: Kameron June 2020Document9 pagesUsing Imagej: Kameron June 2020Stephanie WilliamsNo ratings yet
- Exercises 05 Brightness and ContrastDocument6 pagesExercises 05 Brightness and ContrastKhairul MuzafarNo ratings yet
- MIPlus20 enDocument32 pagesMIPlus20 enIstiaque HaiderNo ratings yet
- Cadi Software ManualDocument8 pagesCadi Software ManualLuis A Gil PantojaNo ratings yet
- Creating HDR PanoramasDocument5 pagesCreating HDR PanoramasCefirel_grifonNo ratings yet
- Lab1 - Manipulating Files in ENVI IDocument12 pagesLab1 - Manipulating Files in ENVI IChainun TaidamrongNo ratings yet
- Hubble Display Manual - 1.24Document8 pagesHubble Display Manual - 1.24juniorsommaiorNo ratings yet
- How To Make A Boot SkinDocument27 pagesHow To Make A Boot SkinsedimbiNo ratings yet
- Photoshop CC 2015 Part 2 Editing and Manipulating Photographs PDFDocument20 pagesPhotoshop CC 2015 Part 2 Editing and Manipulating Photographs PDFharakkNo ratings yet
- Basic Image Processing ImagejDocument9 pagesBasic Image Processing ImagejSg89No ratings yet
- Met 5525 Image Processing Instruction Guide: Shape, Particle Counting and Surface AreaDocument11 pagesMet 5525 Image Processing Instruction Guide: Shape, Particle Counting and Surface AreaMashfiqul IslamNo ratings yet
- Measure Intensity Using Image JDocument4 pagesMeasure Intensity Using Image JJin HoNo ratings yet
- Two Ways To Count Cells With Imagej: Manual Cell Counting and Marking (Plugin Required)Document5 pagesTwo Ways To Count Cells With Imagej: Manual Cell Counting and Marking (Plugin Required)Brunno CaetanoNo ratings yet
- Adobe Photoshop CS4 Part 2: Editing and Manipulating PhotographsDocument21 pagesAdobe Photoshop CS4 Part 2: Editing and Manipulating PhotographsTrevor SelwynNo ratings yet
- Fast Forward With Adobe PhotoshopDocument40 pagesFast Forward With Adobe PhotoshopRona LinNo ratings yet
- ENVI Tutorial: Basic Hyperspectral AnalysisDocument10 pagesENVI Tutorial: Basic Hyperspectral AnalysisPartha GhoshNo ratings yet
- Cms-Rip-Linearization and ProfilingDocument8 pagesCms-Rip-Linearization and Profilingvictor DerrihuNo ratings yet
- Easybuilder Course Lab 5: Color Lab: Colortoolslab - Doc MP 11/21/2015 1Document12 pagesEasybuilder Course Lab 5: Color Lab: Colortoolslab - Doc MP 11/21/2015 1amisculeseiliviuNo ratings yet
- 3Document9 pages3usharani sNo ratings yet
- Arcview Image Analysis: Data For This Tutorial Is Located in The Arcview Installation Directory, Avtutor SubdirectoryDocument30 pagesArcview Image Analysis: Data For This Tutorial Is Located in The Arcview Installation Directory, Avtutor Subdirectorysasa.vukojeNo ratings yet
- Plot Digitizer: User GuideDocument16 pagesPlot Digitizer: User GuideNikola DjordjevicNo ratings yet
- NB2121 Practical 1 ExercisesDocument11 pagesNB2121 Practical 1 ExercisesRushan TanNo ratings yet
- Hyperspectral ENVI ManualDocument22 pagesHyperspectral ENVI Manualry08ty100% (1)
- Tls Data Processing Exploration.v5Document14 pagesTls Data Processing Exploration.v5Dömïñätïñg PratapNo ratings yet
- Echoes in Space SNAP IntroDocument2 pagesEchoes in Space SNAP IntroIgnacio Borlaf MenaNo ratings yet
- Cantilever Beam Model in HypermeshDocument15 pagesCantilever Beam Model in HypermeshgiangfvuNo ratings yet
- V-Ray 1.50.SP2 Tutorial: Creating Depth of Field Blur Effect in Photoshop Using V-Ray 1.50.SP2 Render ElementDocument9 pagesV-Ray 1.50.SP2 Tutorial: Creating Depth of Field Blur Effect in Photoshop Using V-Ray 1.50.SP2 Render ElementIvan Arturo Viloria HermanNo ratings yet
- Stewart Sentinel 1 Processing 179 PDFDocument4 pagesStewart Sentinel 1 Processing 179 PDFMao FonsecaNo ratings yet
- A Beginners Guide To Digital Textile PrintingDocument16 pagesA Beginners Guide To Digital Textile PrintingJessica HibbertNo ratings yet
- Taking Spectral Profiles With FV: A Simple User GuideDocument7 pagesTaking Spectral Profiles With FV: A Simple User GuidePadelisBazanosNo ratings yet
- Object Base Image Classifications BangladeshDocument58 pagesObject Base Image Classifications BangladeshMohd Razif Sumairi100% (1)
- Basic Image Processing Using Envi - Rev1Document23 pagesBasic Image Processing Using Envi - Rev1adi_maniztNo ratings yet
- Classification TutorialDocument35 pagesClassification TutorialrafikscribdNo ratings yet
- Procedure PhotomodeDocument16 pagesProcedure Photomodesharonlly toumasNo ratings yet
- BioImageXD GettingstartedDocument9 pagesBioImageXD GettingstartedAga NowikNo ratings yet
- Lab 2 Radiometric CorrectionDocument5 pagesLab 2 Radiometric Correctionaidatul jannah100% (1)
- Colour Deconvolution: Java and Class FilesDocument10 pagesColour Deconvolution: Java and Class FilesMarianna StasinopoulouNo ratings yet
- Eel 5661 F22 HW4 111122Document4 pagesEel 5661 F22 HW4 111122MohammedNo ratings yet
- Class 6 (Chap 4 & 5)Document5 pagesClass 6 (Chap 4 & 5)druhin.milly2017No ratings yet
- Users Guide MikroCamLab Image ProcessingDocument8 pagesUsers Guide MikroCamLab Image Processingguypaul1No ratings yet
- Learning Activity Sheet Computer Science 2Document17 pagesLearning Activity Sheet Computer Science 2Jaeda BaltazarNo ratings yet
- HDR Images in PhotoshopDocument2 pagesHDR Images in PhotoshopAldrin PaguiriganNo ratings yet
- Unsupervised Classfication Using ER MapperDocument9 pagesUnsupervised Classfication Using ER MapperavisenicNo ratings yet
- Color Display Settings ProcessDocument5 pagesColor Display Settings Processog5sajibNo ratings yet
- Multi SpectralDocument3 pagesMulti SpectralCris SosaNo ratings yet
- Part of Ilustrator: Fills and StrokesDocument3 pagesPart of Ilustrator: Fills and StrokesFara NumeNo ratings yet
- Stationery Quick GuideDocument1 pageStationery Quick GuideSam PevensieNo ratings yet
- Adobe Photoshop: Learn Photoshop In 20 Hours Or Less!From EverandAdobe Photoshop: Learn Photoshop In 20 Hours Or Less!Rating: 3.5 out of 5 stars3.5/5 (6)