Professional Documents
Culture Documents
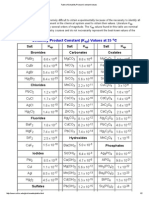
Table of Pka Values
Uploaded by
jVOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Table of Pka Values
Uploaded by
jVCopyright:
Available Formats
TABLE OF pKa VALUES
ACID
CONJ. BASE
H2SO4
OH
HSO4
pKa
-9
CH3CCH3
HCl
Cl
CH3S
-7.3
CH3CCH3
OH
CH3S
-7
-7
H
CH3CH2OCH2CH3
-3.6
CH3CH2OH
CH3CH2OH
-2.4
H 3O
H 2O
-1.7
HNO3
NO3
-1.3
CH3CH2OCH2CH3
H
SOH
SO
-0.6
CCl 3COH
O
CHCl2COH
O
CH2ClCOH
ACID
CCl 3CO
0.64
O
CHCl2CO
1.3
O
CH2ClCO
CONJ. BASE
2.8
pKa
HF
3.2
HCOH
3.7
HCO
O
COH
CO
4.2
CONJ. BASE
pKa
ACID
NH3
OH
NH2
O
CH3COH
CH3CO
4.8
5.2
HCO3
CO32-
6.5
10.2
N H
H2CO3
HCO3
O 2N CH3NO2
OH
CH2NO2
O 2N
SH
7.8
10.5
CH3CH2S
O O
CH3CCH2CCH3
9.0
CH3CCHCCH3
HC N
NH3 4
CH3NH
(CH 3)3NH
H
10.2
7.2
CH3CH 2SH
O
O
4.6
10.0
C N
9.1
CH3NH
NH32
9.4
10.6
9.8
15.0
(CH 3)3N
CH3
O
O
CHO3COH
CH
CH
CCH
CNH
33CH
32CH
CH
CH
H
OOHOH
CH
COCH
3 323 32 2
CH3
OOO
CH3CO
CH
CH
CNH
CCH
3OH
3CH
2
CH
CH
CH
CH
3O
3 2O 2CH2
23COCH
19
19
15.0
17
15.5
23
15.7
ACID
CONJ. BASE
CHCl3
HC
CH
H2C
CH2
CH4
CCl 3
25
CH
26
31
NH2
36
NH3
pKa
H2C
CH
CH3
**Adapted from Organic Chemistry, 2nd Ed., S. Ege, D.C. Heath & Co., 1989.
36
49
You might also like
- McmurryDocument2 pagesMcmurryvinicius oliveiraNo ratings yet
- Physical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976From EverandPhysical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976A. FruchierNo ratings yet
- Chuoi Phan Ung Ve AminDocument2 pagesChuoi Phan Ung Ve AminNguyễn LyNo ratings yet
- Pka ValueDocument6 pagesPka ValueNut ChurinthornNo ratings yet
- Halogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesFrom EverandHalogenated Benzenes, Toluenes and Phenols with Water: Solubility Data SeriesAri L. HorvathRating: 5 out of 5 stars5/5 (1)
- Inborn Errors ChartDocument1 pageInborn Errors ChartGrausamvsNo ratings yet
- The Modern Spanish: Breyer and Zaitsev SystemsFrom EverandThe Modern Spanish: Breyer and Zaitsev SystemsRating: 5 out of 5 stars5/5 (1)
- Ca C Cac: Ca Oh Cao Ho Cao Co CacoDocument3 pagesCa C Cac: Ca Oh Cao Ho Cao Co CacoTam Duong TranNo ratings yet
- Pka ChartDocument2 pagesPka ChartSayNo ratings yet
- Nhung Van de Ly Thuyet THPT Thuong GapDocument126 pagesNhung Van de Ly Thuyet THPT Thuong Gaptuấn cường trần nguyễnNo ratings yet
- Formula Zio ADocument9 pagesFormula Zio AJara EspumosaNo ratings yet
- 355 FC618 D 01Document3 pages355 FC618 D 01Mery BladieNo ratings yet
- Carboxylic AcidsDocument41 pagesCarboxylic AcidsSazzad TanimNo ratings yet
- La Síntesis Total de Colesterol: R. B. WoodwardDocument4 pagesLa Síntesis Total de Colesterol: R. B. WoodwardHector Martinez GregorioNo ratings yet
- So Do Chuoi Cac Phan Ung Hoa HocDocument8 pagesSo Do Chuoi Cac Phan Ung Hoa HoctienduongsatthuNo ratings yet
- Alkylation of Enolate IonsDocument13 pagesAlkylation of Enolate IonsGabriel PekárekNo ratings yet
- Conversions (ORGANIC)Document8 pagesConversions (ORGANIC)Abir Dutta80% (5)
- Common Chemical Formula ListDocument3 pagesCommon Chemical Formula Listaran9280% (5)
- H3C-C-CH2-COOH Acid Acetoacetic O OH CH3-CH-CH2-COOH O Acid - Hidroxi Butiric Acetona H3C-C-CH3Document5 pagesH3C-C-CH2-COOH Acid Acetoacetic O OH CH3-CH-CH2-COOH O Acid - Hidroxi Butiric Acetona H3C-C-CH3Grigoras CorneliaNo ratings yet
- Acizi Carboxilici RO-1-1Document8 pagesAcizi Carboxilici RO-1-1Teo OlteanNo ratings yet
- PS11 S07 SolnDocument5 pagesPS11 S07 SolnJerika ArceoNo ratings yet
- I) Chalcones and Cinnamonyl Derivatives: Archo Piperidine, CHCL, RefluxDocument27 pagesI) Chalcones and Cinnamonyl Derivatives: Archo Piperidine, CHCL, RefluxUjjwal SharmaNo ratings yet
- AlcaniDocument3 pagesAlcanibroscutzatNo ratings yet
- An Incomplete Polyatomic Ion ChartDocument1 pageAn Incomplete Polyatomic Ion ChartGAT TutoringNo ratings yet
- Pka ValuesDocument35 pagesPka ValuesTiago Breve da SilvaNo ratings yet
- Curs NR 10Document21 pagesCurs NR 10Sinziiana SuhareanuNo ratings yet
- 421 Assi-6 SON (Chemi)Document7 pages421 Assi-6 SON (Chemi)Vrushabh IngleNo ratings yet
- Transport Acetil CoADocument1 pageTransport Acetil CoADejan ZolakNo ratings yet
- CHP 18 Answer KeyDocument5 pagesCHP 18 Answer Keyeuler555No ratings yet
- 03 Glycolysis, FermentationsDocument1 page03 Glycolysis, Fermentationsİsmail ŞimşekNo ratings yet
- 12e1 PDFDocument5 pages12e1 PDFwastequestNo ratings yet
- Nmrlec 3Document20 pagesNmrlec 3Abdo AogNo ratings yet
- Glucidele: ClasificareDocument12 pagesGlucidele: ClasificareIoana MunteanNo ratings yet
- Ctes de Solubilidad01Document13 pagesCtes de Solubilidad01SorlisasNo ratings yet
- Pka TablesDocument39 pagesPka TablesRisa R.No ratings yet
- ALKANDocument18 pagesALKANLân Võ ThànhNo ratings yet
- Table of Some Common Polyatomic Ions Formulae Constants and ConversionsDocument1 pageTable of Some Common Polyatomic Ions Formulae Constants and ConversionsEsat GoceriNo ratings yet
- Carbanions IDocument40 pagesCarbanions INurhan KishaliNo ratings yet
- AIEEE Chemistry QuickReviewDocument1 pageAIEEE Chemistry QuickReviewAkshay NilawarNo ratings yet
- Carboxylic Acids:: R-Cooh, R-Co HDocument29 pagesCarboxylic Acids:: R-Cooh, R-Co HRao Wazim AkramNo ratings yet
- Aldehide: Metodele de ObţinereDocument7 pagesAldehide: Metodele de Obţinereisoscel34No ratings yet
- Shenvi Sep 04Document10 pagesShenvi Sep 04patilamardip007No ratings yet
- Carbanions - C: - The Conjugate Bases of Weak Acids, Strong Bases, Excellent NucleophilesDocument40 pagesCarbanions - C: - The Conjugate Bases of Weak Acids, Strong Bases, Excellent NucleophilesSiicwek GeminieNo ratings yet
- Acid Carboxylic Beùo Vaø Daãn ChaátDocument38 pagesAcid Carboxylic Beùo Vaø Daãn ChaátthidaithanhNo ratings yet
- Acid Carboxylic Beùo Vaø Daãn ChaátDocument38 pagesAcid Carboxylic Beùo Vaø Daãn ChaátBích Nguyễn NgọcNo ratings yet
- Curs 11, 12Document29 pagesCurs 11, 12didibutterflyNo ratings yet
- 2Nh 2Nh: KCL Oh Koh CL B Cuso Na S Cus Na So Nicl Na S NisDocument1 page2Nh 2Nh: KCL Oh Koh CL B Cuso Na S Cus Na So Nicl Na S NisLuigi MoralesNo ratings yet
- Non Polar-Hydrophobic-Buried in Protein Core-AliphaticDocument4 pagesNon Polar-Hydrophobic-Buried in Protein Core-AliphaticRob FranciscoNo ratings yet
- Exercícios Resolvidos - Cap. 10 (Ímpares) - Ácidos e Bases - Princípios de Química - AtkinsDocument40 pagesExercícios Resolvidos - Cap. 10 (Ímpares) - Ácidos e Bases - Princípios de Química - AtkinsJaoJaoNo ratings yet
- Top All27 Prot LipidDocument89 pagesTop All27 Prot LipidJose CastroNo ratings yet
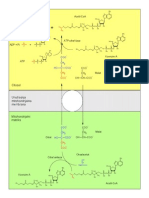
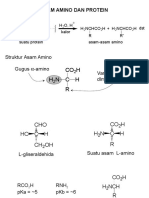
- Bab 19. Asam AminoDocument58 pagesBab 19. Asam AminoSisca Ayu VerawatiNo ratings yet
- R-Cooh, R-Co H,: À Ant À VinegarDocument43 pagesR-Cooh, R-Co H,: À Ant À VinegarArvin MarasiganNo ratings yet
- Table of Solubility Product ConstantDocument2 pagesTable of Solubility Product ConstantIqbal Wardani100% (1)
- Problem Set No. 5Document12 pagesProblem Set No. 5Sofia DalisayNo ratings yet
- Table PkaDocument1 pageTable PkaKhánh Nguyễn Trần BáNo ratings yet
- Amino Acid Abbreviations Molecular Formula Linear FormulaDocument1 pageAmino Acid Abbreviations Molecular Formula Linear FormulaBeatrix RossNo ratings yet
- Organic Tute 8Document7 pagesOrganic Tute 8isuri sewwandiNo ratings yet
- ElaborationDocument1 pageElaborationdr.igayenNo ratings yet
- Psyc 281Document6 pagesPsyc 281jVNo ratings yet
- GPA GPA Credits NEW Credits: Term GPA Req'DDocument4 pagesGPA GPA Credits NEW Credits: Term GPA Req'DjVNo ratings yet
- Emergency Action Plan For Runathon 2015Document8 pagesEmergency Action Plan For Runathon 2015jVNo ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Phase Equilibria in Chemical EngineeringFrom EverandPhase Equilibria in Chemical EngineeringRating: 4 out of 5 stars4/5 (11)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)