Professional Documents
Culture Documents
No 28
Uploaded by
Louie Shaolin Lungao0 ratings0% found this document useful (0 votes)
8 views2 pagesi
Original Title
no.28
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documenti
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
8 views2 pagesNo 28
Uploaded by
Louie Shaolin Lungaoi
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
The data in the table have been obtained on the decomposition of gaseous reactant
A in a constant volume batch reactor at 100 degrees Celsius. The stoichiometry of
the reaction is 2A ------R + S. What size plug flow reactor(in liters) operating at 100
degrees Celsius and 1 atm can treat 100 mol A/hr in a feed consisting of 20% inert
to obtain 95% conversion of A?
t,sec PA, atm
0 1.00
20 0.80
40 0.68
60 0.56
80 0.45
100 0.37
140 0.25
200 0.14
260 0.08
330 0.04
420 0.02
Given: CVBR
100mol/hr
20% inert
100 C 1 atm
Xa=0.9
Required: V(L) of plug flow reactor
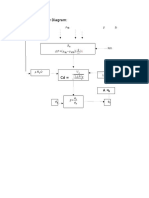
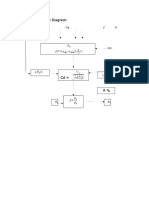
Solution:
CVBR
Assume first order reaction
kt = -In(1-XA)
A= 0
Fao= 100(100/80)
Pao=0.8 atm
K=
0.011 0.009 0.009 0.01 0.009 0.009 0.009 0.009 0.009 0.009
2 6 7 9 9 8 7 8 3
K= 0.01/sec
In plug flow reactor
V/Vo= -In(1-Xa)/k
Vo=(FAoRT)/Pao
= (100)(0.08205)(373.15)/0.8
= 3827.12 L/hr
= 1.06 L/sec
V= (1.06)(-In(1-0.95))/0.01
= 317.55L
You might also like
- Chapter IV and VDocument2 pagesChapter IV and VLouie Shaolin LungaoNo ratings yet
- Laplace Transforms for ODE SolutionsDocument20 pagesLaplace Transforms for ODE SolutionsJamel CayabyabNo ratings yet
- IntroductionDocument3 pagesIntroductionLouie Shaolin LungaoNo ratings yet
- The Laplace TransformsDocument61 pagesThe Laplace TransformsLouie Shaolin Lungao100% (1)
- No 28Document2 pagesNo 28Louie Shaolin LungaoNo ratings yet
- Interpretation of Results and ObservationsDocument1 pageInterpretation of Results and ObservationsLouie Shaolin LungaoNo ratings yet
- Information Flow DiagramDocument1 pageInformation Flow DiagramLouie Shaolin LungaoNo ratings yet
- Ilocanos: "Kuripot O Matipid?" #Ilocandia: Submitted ToDocument21 pagesIlocanos: "Kuripot O Matipid?" #Ilocandia: Submitted ToLouie Shaolin LungaoNo ratings yet
- Chapter IV and V 2Document2 pagesChapter IV and V 2Louie Shaolin LungaoNo ratings yet
- Chapter 4 Expt 4Document4 pagesChapter 4 Expt 4Louie Shaolin LungaoNo ratings yet
- Case Study ChernobylDocument4 pagesCase Study ChernobylLouie Shaolin LungaoNo ratings yet
- Expt 05 ReportDocument18 pagesExpt 05 ReportLouie Shaolin LungaoNo ratings yet
- Chapter 2. Expt IronDocument1 pageChapter 2. Expt IronLouie Shaolin LungaoNo ratings yet
- VenturimeterDocument8 pagesVenturimeterAbhishek Kandoi100% (1)
- Expt 05 ReportDocument18 pagesExpt 05 ReportLouie Shaolin LungaoNo ratings yet
- Information Flow DiagramDocument1 pageInformation Flow DiagramLouie Shaolin LungaoNo ratings yet
- Experiment 1 REPORTDocument18 pagesExperiment 1 REPORTLouie Shaolin LungaoNo ratings yet
- NulllllaasaDocument1 pageNulllllaasaLouie Shaolin LungaoNo ratings yet
- Information Flow DiagramDocument1 pageInformation Flow DiagramLouie Shaolin LungaoNo ratings yet
- JHVJHDocument1 pageJHVJHLouie Shaolin LungaoNo ratings yet
- JHVJHDocument1 pageJHVJHLouie Shaolin LungaoNo ratings yet
- No.28 and 29Document2 pagesNo.28 and 29Louie Shaolin LungaoNo ratings yet
- Example PolymerDocument2 pagesExample PolymerLouie Shaolin LungaoNo ratings yet
- Results and DiscussionDocument4 pagesResults and DiscussionLouie Shaolin LungaoNo ratings yet
- NulllllDocument1 pageNulllllLouie Shaolin LungaoNo ratings yet
- Coating FormulationDocument2 pagesCoating FormulationLouie Shaolin LungaoNo ratings yet
- NULLDocument1 pageNULLLouie Shaolin LungaoNo ratings yet
- NULLDocument1 pageNULLLouie Shaolin LungaoNo ratings yet
- NULLDocument1 pageNULLLouie Shaolin LungaoNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (894)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)