Professional Documents
Culture Documents
Chenchen Fan Project Small
Uploaded by
api-367780038Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chenchen Fan Project Small
Uploaded by
api-367780038Copyright:
Available Formats
Contrasting morphologies of low and high elevation populations of the cinnabar moth (Tyria jacobaeae) in Oregon
Chenchen Fan, Monte Mattsson, Linda Buergi, Peter Mcevoy
Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR
Introduction
The cinnabar moth, Tyria jacobaeae (Lepidoptera: Erebidae), is endemic from Europe through
central Asia, and in the last century has been introduced by humans to New Zealand, Australia and
North America as a biocontrol agent for the invasive noxious weed tansy ragwort, Jacobaea vulgaris
Results
(Asteraceae), on which its larvae feed. Populations of the moth were introduced to the Willamette MASS
Valley, Oregon beginning in 1960 (Frick and Holloway 1964). Throughout the 1980s, Oregon I found no significant difference in adult mass between mountain and valley origin moths (F1,103=0.32, P=0.58), but a
populations of the moth were further redistributed at numerous sites in the Cascade Mountains to clear difference between female and male moths (F1, 103=39, P<0.001) (Figure 2). The interaction between origin and
control emergent tansy ragwort populations following clear-cut logging and forest fires (Coombs sex was not significant (F1,102 = 1.1, P=0.29)
1996). Redistribution efforts ceased around 1985, therefore mountain and valley populations of the WING LENGTH
cinnabar moth have been genetically isolated for ~30 years. For wing length, I found no significant difference between mountain and valley origin moths (F1,103=1.48, P=0.23),
but a clear difference between female and male moths (F1, 103=39, P<0.001) (Figure 3). The interaction between origin
and sex was not significant (F1,102 = 0.40, P=0.53).
WING LOADING (= WING LENGTH/WEIGHT RATIO)
Goals The interaction between origin and sex was marginally significant (F1,102 = 2.88, P=0.09), suggesting a sex specific effect of
origin. When analyzed separately, mountain male moths had higher wing loading compared to valley male moths (t=-1.87,
In this study, I test hypotheses relating to wing morphology and body mass using adult cinnabar moths from both Cascade Mountains
P=0.07), and no difference in female moths (t=-0.11, P=0.9) (Figure 4).
(elevation: ~1500 m) and Willamette Valley, OR (elevation: ~50 m) environments. Moths might have different wing sizes due to different
abiotic forces driving adaptation. I hypothesize that the mountain moths have larger wing area to body weight ratio, because they have to fly in
the thinner air in the higher elevation mountain where atmospheric pressure is lower than in the valley region. In most moth species size and
need for flightiness differs significantly between the sexes, so I hypothesize that any wing loading differences we would find might differ
between male and female moth. I was able to test if (1) mountain and valley moths had similar body mass; (2) male and female moths had
similar body mass; (3) mountain and valley origin moths had similar wing length; (4) male and female moths had similar wing lengths; and (5)
there was an interactions between sex and geographic origin for any of the traits under study.
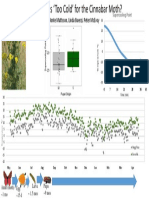
Cascade Mountains Willamette Valley, OR
(Elevation: 1572 m) (Elevation: 87 m)
http://www.summitpost.org/images/original/790903.JPG
http://media-cache-ak0.pinimg.com/736x/97/f1/36/97f136f3a75e0d7a6245c5c5056e2895.jpg Figure 2 Figure 3 Figure 4
Figure 2: Body mass of females (left) and males (right) from mountain (M) and valley (V) environments.
Figure 3: Wing lengths of female (left graph) and male (right graph) moths from mountain (M) and valley (V) environments.
Figure 4: Wing lengths of female (left graph) and male (right graph) moths from mountain (M) and valley (V) environments.
Materials and Methods
The cinnabar moth larvae were collected in 2015 and reared in the laboratory until
pupation. Mountain- and valley-origin pupae were overwintered in a common, low
elevation environment. In spring 2016, the pupae were brought back into the lab and
monitored adult eclosion daily. Then cinnabar moths were photographed, weighed,
and sexed upon eclosion. Then ImageJ (Schneider, Rasband and Eliceiri 2012) was Discussion
used to analyze the moth images. To avoid bias, I was unaware of the moths origins My hypothesis of increased wing loading in mountain moths has been confirmed with marginal significance for male
while performing measurements. To transform units between pixels and metric scale moths. Female moths did not show any difference in wing loading. In the cinnabar moth females are often too heavy to fly right after
measuremetns we included a ruler in each picture. Then, 105 moths were selected for emergence due to large egg loads. The females remain close to the site of emergence and attract the more mobile males. Thus, it makes
data analysis, comprised of 17 mountain origin males and 34 mountain origin females, sense that males should be the ones that have adapted to the difference in flight condition. As seen in the adult weight, wing length and
19 valley origin males and 35 valley origin females. When selecting which wing to wing length/ adult weight relationship, males tend to be lighter with larger wings, a combination leading to increased flight ability of the
measure, I alternated between left and right wing depending upon which was clearer in males compared with the females.
in the image. Measurements included (1) wing length, measured as distance from head Figure 1 Future studies could address this question with an increased sample size and possibly dissect the wings off the moths rather
to the most distal point on the wing (Figure 1), (2) body mass. than taking pictures of live moths, where wing angles and positions lead to a larger measurement error. In addition, future studies should
determine if the changes observed in the mountain males are adaptive, leading to higher fitness in the local environment. Second, it should
be investigated what these changes are adaptive to with our main hypothesis being thinner air at higher elevation, but other possibilities
including patchier hosts or windier environment.
Reference
Coombs, E.M., H. Radtke, D. L. Isaacson, and S.P.Snyder. 1996. Economic and regional benefits from the biological control of tansy ragwort,
Senecio jacobaea, in Oregon. In Proceedings of the IX International Symposium on Biological Control of Weeds, pp. 489-494.
University of Cape Town, South Africa.
Frick, K.E., and J.K.Holloway 1964. Establishment of the cinnabar moth, Tyria jacobaeae, on tansy ragwort in the western United States.
Journal of Economic Entomology 57: 152-154.
Schneider, C.A.; Rasband, W.S. and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis, Nature methods 9(7): 671-674,
PMID 22930834
You might also like
- Katy Berns PosterDocument1 pageKaty Berns Posterapi-367780038No ratings yet
- Spencer Gravitt PresentationDocument1 pageSpencer Gravitt Presentationapi-367780038No ratings yet
- Baylee Mayfield PresentationDocument1 pageBaylee Mayfield Presentationapi-367780038No ratings yet
- Chenchen Fan Project SmallDocument1 pageChenchen Fan Project Smallapi-367780038No ratings yet
- Tim Lieberenz ProjectDocument1 pageTim Lieberenz Projectapi-367780038No ratings yet
- Wade Johnson Project 1pageDocument1 pageWade Johnson Project 1pageapi-367780038No ratings yet
- Project Poster-Kelsey WoolseyDocument1 pageProject Poster-Kelsey Woolseyapi-367780038No ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Ideal Vs Real OttoDocument5 pagesIdeal Vs Real Ottoa7med SoulimanNo ratings yet
- Evolis User ManualDocument28 pagesEvolis User ManualIonmadalin1000No ratings yet
- Results 2020: Climate Change Performance IndexDocument32 pagesResults 2020: Climate Change Performance IndexTonyNo ratings yet
- Union Metal SemiconductorDocument4 pagesUnion Metal SemiconductorskinhugoNo ratings yet
- Nokia 2690 RM-635 Service ManualDocument18 pagesNokia 2690 RM-635 Service ManualEdgar Jose Aranguibel MorilloNo ratings yet
- MHSS ItalyDocument9 pagesMHSS Italyromedic36No ratings yet
- Si2304 (Mosfet Sot 23)Document6 pagesSi2304 (Mosfet Sot 23)Alfredo Valencia RodriguezNo ratings yet
- Haberman Data Logistic Regression AnalysisDocument5 pagesHaberman Data Logistic Regression AnalysisEvelynNo ratings yet
- The Research Problem: The Key Steps in Choosing A TopicDocument5 pagesThe Research Problem: The Key Steps in Choosing A TopicJoachim San JuanNo ratings yet
- Validation For A Login PageDocument2 pagesValidation For A Login PageAmal RajNo ratings yet
- PP in Ii 001Document15 pagesPP in Ii 001Dav EipNo ratings yet
- Chapter 15 - Leukocyte Migration and Inflammation - The IS Relies Upon The Continual Circulation of Leukocytes Through The BodyDocument12 pagesChapter 15 - Leukocyte Migration and Inflammation - The IS Relies Upon The Continual Circulation of Leukocytes Through The BodyEmad ManniNo ratings yet
- FDocument102 pagesFTop channelNo ratings yet
- Basic Electrical Safety Module 1Document39 pagesBasic Electrical Safety Module 1malawi200No ratings yet
- Bibliography and FootnotesDocument2 pagesBibliography and FootnotesHannah de VeraNo ratings yet
- One Plan Student 1Document7 pagesOne Plan Student 1api-465826207No ratings yet
- Bike Share ReportDocument16 pagesBike Share Reportsanjay975100% (1)
- Sect. 4 Tech Docum PC7 AutoLube - 1209 PDFDocument46 pagesSect. 4 Tech Docum PC7 AutoLube - 1209 PDFAlexis MikeNo ratings yet
- SOL-Logarithm, Surds and IndicesDocument12 pagesSOL-Logarithm, Surds and Indicesdevli falduNo ratings yet
- Hamming Code - Error Detection Aim: AlgorithmDocument12 pagesHamming Code - Error Detection Aim: Algorithmkrithikgokul selvamNo ratings yet
- PUP 200 Quizzes 6Document47 pagesPUP 200 Quizzes 6Nam TranNo ratings yet
- IMG - 0009 Thermodynamic Lecture MRCDocument1 pageIMG - 0009 Thermodynamic Lecture MRCBugoy2023No ratings yet
- Revised Research ZoomDocument51 pagesRevised Research ZoomAubrey Unique EvangelistaNo ratings yet
- Analytical Chemistry (CHM111) Laboratory ManualDocument73 pagesAnalytical Chemistry (CHM111) Laboratory ManualKatrina BucudNo ratings yet
- Template 3 - MATH 3-REGULAR-DIAGNOSTICDocument2 pagesTemplate 3 - MATH 3-REGULAR-DIAGNOSTIClailanie CervantesNo ratings yet
- Chapter 1 MPLS OAM Configuration Commands ...................................................................... 1-1Document27 pagesChapter 1 MPLS OAM Configuration Commands ...................................................................... 1-1Randy DookheranNo ratings yet
- Power Distribution & Utilization: Total Power Generation of Last 10 Years and Forecast of 20 YearsDocument12 pagesPower Distribution & Utilization: Total Power Generation of Last 10 Years and Forecast of 20 YearsSYED ALIYYAN IMRAN ALINo ratings yet
- Structure and Operation: 3. Electronic Control Unit Connection DiagramDocument16 pagesStructure and Operation: 3. Electronic Control Unit Connection DiagramAung Hlaing Min MyanmarNo ratings yet
- NeedScope On TechnologyDocument22 pagesNeedScope On TechnologyNguyen Ngo Dinh PhuongNo ratings yet
- Blockchain Disruption in The Forex Trading MarketDocument64 pagesBlockchain Disruption in The Forex Trading MarketVijayKhareNo ratings yet