Professional Documents
Culture Documents
Alcohols, Ethers, Thiols, and Sulfides
Uploaded by
nspegar920 ratings0% found this document useful (0 votes)
10 views6 pagesAlcohols, Ethers, Thiols, and Sulfides
Original Title
Alcohols, Ethers, Thiols, And Sulfides
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentAlcohols, Ethers, Thiols, and Sulfides
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views6 pagesAlcohols, Ethers, Thiols, and Sulfides
Uploaded by
nspegar92Alcohols, Ethers, Thiols, and Sulfides
Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 6
4 nk
Dritial Concpls & Reveur Problens
v
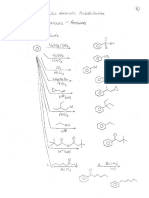
; Organic Chem Alcohols / ethers / Chis /sulfides
le OL Name tg: Bea dbeucteres
OH
a. 6)
; Coad preseryctive) 2H (menthol)
: flavoe
B-
2 4 ret
@) tis — 2-Usobu: cyclohexanol
§ trons ~3- chloro eyclopentanol
& 4 ~phengh— 1,5 — —heranediol
2, Shou the reaction 4 crc -molecale prom pro sblems
tothe KG Oy oxide zing reagent, Showd Be nat
tarrick produc § no, action” may. he a gessbee
5 ans, Sor diols. eat both, alcohat gecge
A Sheu the reaction of tack, tolecale proms problem, */
(with. He S04 tebalys€ aa & debe dara ton reagent,
Shaw both products 4 pick major product cf there.
(OS one, Ser the diols, two ene
| apply the reaction to bath, akcohst
Cisne wantion’ meg be a possible anstsas)
| (Orly Ewe products par the Lidls since one y the
| alenhols cen produce only ss pocsithe foulke bond)
2@
Ou
koa YOK 2,6 dhe - ene -butyd - 4-metigh-
3
se sphenoh
&. tod 2-tsopropye — t- methyl eqclohexanoL
8 -broimo -
S~(S -Lsepropykegeloher ye)~
Alo ~ dimethek ~3-nonanok
S— propyl LA octaneddok
OW
2) oH £) g) HOLA
Crk , s 8 a
ee
2a) no reaction
- b) K.Cr, O4
: weet Lo
‘ OH
™~
8.
j K2Cr0.
Q! ~
a OH ~Y
4) te Keng Sot HO A
| ©
| He pH © no stereacher.!
Me fe eso, Oye *
2H ee Oo + 8
a ce ORD
oO He so, ®
® wtP i we 280 ®
Zo .
a
1 OA? pL
ALLS
QPEL
Strplet tyclopentyl Replyk ether
systematic: I> cyclopentonsheptone Cor heptorg eyclopentane)
1 ha,
by, PS Ay S-chloro#~ proporg ~ 1,-octaduene
O
Oke hig 2 , Hoehlore -®-athye-8-
1
® T heptanethcok
bo ONAL batys phenyh suri,
Sa). “NON moderati: poloee
on = strong Lipol: wv Rayhrogen bonding hegher 8. P
* shong dipole w H- Gonding mech etm ane mora
6) NN ont ¥ Hagler ®.PY both “hans Sea
| on force 44008
| When. molecates hae Sout fare types Highr MM “hig BP
8 oe strong dipole w H bonding
Oesu = moderate Aipole
@
OH Lo Ce ‘\ stereochemestieg.
2.2) Kke0y ho. longer ey
wn meet,
puny \( teigonal planar ()
i Keno, “
3. &) hovulin
6) He H, 2804 gs «
b H SN OH
(ase neigh boeing HS ave Shown)
~ Mat
aS imap.
Ogee
He Ha
5 et 30, © DO maj
RL 8s CON SLE
+
oR OL
no storeschent Ceigel iplanor (
H2S0q |
b) eS majo be | on
ruleas qeven.
@
1 Nene a) Drone C name by single method
: 9 schematic metho)
a “
by, wa-r 9 “yh
me SH
a) cero Cstmpte me that!)
S. Whack, gf te ftw0 Congouende has higher hocking Poca
4) re BW oy 2 Coot * Same Mam)
by Sok ge OH t (erg Actforenk 4)
Sy crn" on Ousa s lboth x Same Mm)
b. Fou a,b Fe from problems, aeleck ihcch, % Hee
theo Compounds is more doluble in water,
4.) Draw tee dteccture $ name the 2fher formed
3~ pentansd plaw |~ brome butane (in baele solution)
b) Gear steuckwes | ndme the three others whic con
~ he foemel en a Solution A Q~ prath ethane
tort (= butenet Cplua aecd catty st) *Gemple sq stem)
2, Shao a two slep Syntheais (2 reactors) fr, Leck 2
2. 3 2.8
a SS SN by Hats cH, —* CHy¢~0H
gre an a om ag
®
bie hen beet solability s-matok, force types (See *s)
Hao = yn, = steong dipole uy tg drogen boading
BOK matches N20 the beaut ~ more soluble
“Sou fee less non-met che tg deoescecn portion
has ts move soluble us BOB
“) cr matches HeO the beet = more soluble
©
@,
si! 2 4 @
14 oS o2e2@ > Aho eo
a XR 8 +
OH
© . 6 <0 an butory pentane
bye OAD
Oy , lL dink a= Cagle inteye ethyl) ether
umrou | Cr@ ONAN
boxy 2 ~egle pentyeethire ether
Q+® ON
. dhbutyt ether
: ‘ OH :
ea ee HS UL ete, RL
: +
iy Heacn, HEE? pe crkou HO Toy
3504
i Ho
eyo Gee TY pe
4 vA et AA Be abo
: Y tee OG 7 Ts,
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Mathnasium Application - Mubashir Razvi PDFDocument2 pagesMathnasium Application - Mubashir Razvi PDFnspegar92No ratings yet
- Carboxylic Acids and Carbonyls PDFDocument10 pagesCarboxylic Acids and Carbonyls PDFnspegar92No ratings yet
- Chem 247 Enols and Nomenclature PDFDocument9 pagesChem 247 Enols and Nomenclature PDFnspegar92No ratings yet
- Organic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFDocument9 pagesOrganic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFnspegar92No ratings yet
- Organometallics, Epoxides, and Ethers Practice PDFDocument6 pagesOrganometallics, Epoxides, and Ethers Practice PDFnspegar92No ratings yet
- Electrophic Aromatic Substitution Practice PDFDocument5 pagesElectrophic Aromatic Substitution Practice PDFnspegar92No ratings yet
- Electrophilic Addition Practice 1 PDFDocument4 pagesElectrophilic Addition Practice 1 PDFnspegar92No ratings yet
- General Reactions Practice PDFDocument5 pagesGeneral Reactions Practice PDFnspegar92No ratings yet
- Dienes and Diels-Alder Practice PDFDocument4 pagesDienes and Diels-Alder Practice PDFnspegar92No ratings yet
- Chem 247 Aromatics and Electrophilic Aromatic Substitution PDFDocument9 pagesChem 247 Aromatics and Electrophilic Aromatic Substitution PDFnspegar92No ratings yet
- Chem 247 Organometallic and Grignard Reactions PDFDocument5 pagesChem 247 Organometallic and Grignard Reactions PDFnspegar92No ratings yet
- Chem 247 Alcohol Redox, Epoxides, and Ethers PDFDocument9 pagesChem 247 Alcohol Redox, Epoxides, and Ethers PDFnspegar92No ratings yet
- Chem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFDocument3 pagesChem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFnspegar92No ratings yet
- Chem 247 Alkynes, Conjugation, and Diels-Alder PDFDocument7 pagesChem 247 Alkynes, Conjugation, and Diels-Alder PDFnspegar92No ratings yet
- Alkyl Halides Practice PDFDocument9 pagesAlkyl Halides Practice PDFnspegar92No ratings yet
- Aromatic Nomenclature and Concepts PDFDocument3 pagesAromatic Nomenclature and Concepts PDFnspegar92No ratings yet
- Aldehydes and Ketones Practice PDFDocument9 pagesAldehydes and Ketones Practice PDFnspegar92No ratings yet
- Chem 247 Exam 3 PDFDocument3 pagesChem 247 Exam 3 PDFnspegar92No ratings yet
- Alkynes Practice PDFDocument3 pagesAlkynes Practice PDFnspegar92No ratings yet