Professional Documents
Culture Documents
Chem 247 Organometallic and Grignard Reactions PDF
Uploaded by
nspegar920 ratings0% found this document useful (0 votes)
19 views5 pagesOriginal Title
Chem 247 Organometallic and Grignard Reactions.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
19 views5 pagesChem 247 Organometallic and Grignard Reactions PDF
Uploaded by
nspegar92Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 5
Organic lt: Extra Problem Set 3
Organometallic Reactions; Grignard Reactions
Follow the guidelines stated in Extra Problem Set 1 to continue to teach yourself the
important procedures for problem solving.
The problems from another text are listed in numerical order based on the text chapter
Configuration, Answers to the problems are included on separate pages a he back
Nofe that some problems included in a particular problem section may
referred to for topics covered /ater. Problems 14.17 (n}, 14.21 (b), and a .24 in this
problem should be solved with Extra Problem Set 6.
Preparation and Properties of Organometallic Compounds;
Nucleophilic Addition and Addition/Elimination: Alcohol Formation from Carbonyls
Organo-zinc and Organo-mercury Etectrophilic Addition Reactions
Problems (all concepts): 14.15, 14.17 - 14.24, 14.26,14.27, 14.29
Try 14.17 {n), 14.21 (b), and 14.24 with Extra Problem Set 6.
(AA.15 Suggest appropsiate methods for preparing each of the following compounds from the sart- mas
ing material of your ctoce DAV The
(2) CHCH,CHCH,CHMgT (9 CHCHCHCHACHL aa
@) CHCH.C=CM gl HO cHoncHcHOLcLi — B) “Scagd + CHaMgh >
Dvn Sb
ra: Wits the sirctreof the principal onpaic product of rach of the fullowing reactions: ”
~ (©) Bromopropane wits liam in diel ether
(©) 1-Bromopropane with magnesium in diethyl ether CL) 7 he
(6 ‘odopropane with ithium in diethyl eter & mn, ger
(6 Dindopogane wih maps in detyt aber) EB 8) Aa Bs
{@) Product of part (a) with copper) iodide «= 2) UN Chi 9) .
(0 Pret of pt) wi oad EE BY AA By) EI
(@) Product of par (@) with iodobenzene = 42.) 7D
() Prove of par (2) with D,O and DCL 5 A 4) Aon
Product of pare (¢) with D0 and DCT 4 oH
) Product of par (a) with formaldehyde in eter, followed by dite acid R) e D Or
(8) Product of prt (0) with benzaldehyde in ster, followed by dite acd
), Product of par (¢) with eycloheptanone in ether, followed by dilute acié
3 rm) ”
(0m) Product of part (¢) with CHsCCHCHs in ether, followed by dilute acid
9
1
(0) Prodvt of pre) with CHsCOCH, (2 mal) in ete, flowed by ate acit ©)
(©) LOctene with disdomethane and zinc-copper couple in ether PE oa ss owt ng CH
KK
(@) (2)-2-Decene with diiodomethane and zine-copper couple in ether
(@) @3-Decene with diodomethane and zine—copper couple in ether
7.38 Using omens an any mesa cage spe fet ya Lig
TSS RE mt ad any cesar ran inane age pp icienteyms — O.) AA. wher
@ PRewmoi (©) 3 Metny--heptaol 1. Ceo
(©) 2Hexanot (©) 1-Butyleyclobutasol Zc ASIO Be
(©) PbeayL- 1 pentanal \
ise tagrecnnt tyne wpe gan napeacaesn ©) ~~ ppg “Sein
thes fen of lowing a Tae
(@) BemsyLatcbol “YS Seg {aot (@) 1-Phenyht-hexanol ©
pe ) AAO feo EY
€ Brasdiphnyntne {09 tmertsioin | eaptecs
{siomnec 5 © tenet Peyote eat i.
“20 Analyze the following structures so as wo determine sll the practical combinations of Grig- t ge
‘atone lk pom sy gemntemsiumetees if SE
@ oe (@) 6Methyl-S-heptea2al . a Had
feck p i
A page L NIB Lee
g
apple eee =
oe wt °
(0 (@i),CCH.OH 4) ~Siiggr bk
avo
1428 Addition of phenylmsgnesiam bromide to 4-ter-btyicyclobexanone gives two isomeric ex-
{iy slobols as product Boh lho ld to same lene wien sjee o acif-aaly 5
Seton Sopp resomble srocwes free we sod ye eg NO axon cccts}g
on.
oo
A ‘teere-Boyglobeancne
maze
i
fonmate (HCOCH,CH,) yields a different class of alcohols on restment with Grignard
‘eagents. What kind of alcohol is formed in this cxse and why?
I
(b) Diethyl carbonate (CHjCH,OCOCH,CH;) reacts with excess Grignard reagent to yield ;
‘cobs ofa pasicolar type. Wat i the sructua felue that characterizes alcohols 1 orngs
‘Prepared in this way? —,
5. Double ester givee alcofate with £9. ~O7 oS SD
<™O7 NON thre idemhcak erie grows Pa
o{¢ 1426 Suggest reasonable stuctares for compounds A, B,,and C in the following reactions: SEe
3} ‘!
3
OTe ican,
(cx LJ O™ PAS compound A + compound Bp .
(as (Cuz) Coty BS Gud, eis
% ee
sn (ors ny fa
ccunse compound B + compound C
(Can)
Compound C is more stable than compound A. OTs stands for tluenssulforat, = DB = E2 product
(eae
14.27 ‘The following conversion has been reported in the chemical literature. It was casied oot in
two steps, tbe first of which involved formation of a p-toluenesuifonate ester. Indicate the reagents
for this step, and show how you could convert the p-toluenesulfonaze to the desired product.
LO Tstl 2
Kon AAS
Geren,
1429 Dipherolmethane i significantly more acidic than benzene, and tiphenylmetane is more
acidic han either Mentfy tbe most acidic proton in esch compound, and suggest a reason for the
trend in seily:
Ci (Colig.Cth, (Cell sCH
Bearene,, Diphenylmetbare
nee Re 10
oy ®
CTS
fake henee tes
form Otani
Bier is Steer Lieed ky ¥
dbth, foen pine ml v
rela
14, 20°
9.
b
4
=)
5
2)
ie ~ ~~
et. Oe OS
£5) 1. Ba He
Brig CH CH (cH),
HG —CHe CHGHS)
i
2
Exum Gs) e-cHeot
t lg oitig Be + HEH = CH),
Bolg eae, seg Grae e ig
5
Fo 5
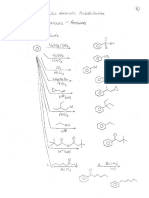
A421 A number of érugs are prepared by reactions of the type described in this chapter. Indicate
what you belie would bea reatonable last sep inthe steals of eats of te folowing. LHeseNa
on
—_—__
) cHycayocence Meparfynol,¢ mild hypoatic or secppiaducing agent 4) _¢ 2. K20
bn . 98,
g -Taeey
r > Grgiees, 3.40
ws catge)tntpmt natin nn eos
of or Hig S-
maple.
hh \CH,O" with aldehde
“1422 Predict the principal organic product of cach of the following reactions: OH
i :
1 2. gid ai CF
@ - + NaCency + 4Etaeete, Cc
> ~<) 7H i 2
CH
w HE) - as .
on
nen
Meme, ca
omy
ake
cHcR—cR, nee He
eich
. 4 cH
nm eal Pe
© Nome’ "St &)y cHe 4
_y
14 Licw(cHy, > Saty
en
is
a
One SN
% ©
cud
e Ham CHACHCH,
a @ sat Scr + LiCu(CH,CH,CH:CHs), —> os
od a
o
=OTS
You might also like
- Organic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFDocument9 pagesOrganic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFnspegar92No ratings yet
- Mathnasium Application - Mubashir Razvi PDFDocument2 pagesMathnasium Application - Mubashir Razvi PDFnspegar92No ratings yet
- Carboxylic Acids and Carbonyls PDFDocument10 pagesCarboxylic Acids and Carbonyls PDFnspegar92No ratings yet
- Organometallics, Epoxides, and Ethers Practice PDFDocument6 pagesOrganometallics, Epoxides, and Ethers Practice PDFnspegar92No ratings yet
- Electrophilic Addition Practice 1 PDFDocument4 pagesElectrophilic Addition Practice 1 PDFnspegar92No ratings yet
- Chem 247 Enols and Nomenclature PDFDocument9 pagesChem 247 Enols and Nomenclature PDFnspegar92No ratings yet
- Chem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFDocument3 pagesChem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFnspegar92No ratings yet
- General Reactions Practice PDFDocument5 pagesGeneral Reactions Practice PDFnspegar92No ratings yet
- Electrophic Aromatic Substitution Practice PDFDocument5 pagesElectrophic Aromatic Substitution Practice PDFnspegar92No ratings yet
- Chem 247 Aromatics and Electrophilic Aromatic Substitution PDFDocument9 pagesChem 247 Aromatics and Electrophilic Aromatic Substitution PDFnspegar92No ratings yet
- Dienes and Diels-Alder Practice PDFDocument4 pagesDienes and Diels-Alder Practice PDFnspegar92No ratings yet
- Alkynes Practice PDFDocument3 pagesAlkynes Practice PDFnspegar92No ratings yet
- Aromatic Nomenclature and Concepts PDFDocument3 pagesAromatic Nomenclature and Concepts PDFnspegar92No ratings yet
- Chem 247 Alcohol Redox, Epoxides, and Ethers PDFDocument9 pagesChem 247 Alcohol Redox, Epoxides, and Ethers PDFnspegar92No ratings yet
- Alcohols, Ethers, Thiols, and SulfidesDocument6 pagesAlcohols, Ethers, Thiols, and Sulfidesnspegar92No ratings yet
- Chem 247 Alkynes, Conjugation, and Diels-Alder PDFDocument7 pagesChem 247 Alkynes, Conjugation, and Diels-Alder PDFnspegar92No ratings yet
- Aldehydes and Ketones Practice PDFDocument9 pagesAldehydes and Ketones Practice PDFnspegar92No ratings yet
- Chem 247 Exam 3 PDFDocument3 pagesChem 247 Exam 3 PDFnspegar92No ratings yet
- Alkyl Halides Practice PDFDocument9 pagesAlkyl Halides Practice PDFnspegar92No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)