Professional Documents
Culture Documents
Chem 247 Alcohol Redox, Epoxides, and Ethers PDF
Uploaded by
nspegar920 ratings0% found this document useful (0 votes)
14 views9 pagesOriginal Title
Chem 247 Alcohol Redox, Epoxides, and Ethers.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
14 views9 pagesChem 247 Alcohol Redox, Epoxides, and Ethers PDF
Uploaded by
nspegar92Copyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 9
@
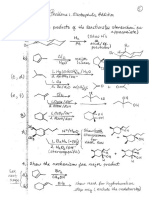
Organic il: Extra Problem Set 4
Alcohol Redox Reactions; Epoxides; Ethers
Follow the guidelines stated in Extra Problem Set 1 to continue to feach yourself the
important procedures for problem solving.
The problems from another text are listed in numerical order based on the text chapter
configuration. Answers to the problems are included on separate pages at the back.
Note that some problems included in a particular problem section may be
referred to for topics covered later. Problems 15.28 and 15.29 in this problem set
include problems to be solved with Extra Problem Set 6; use the topic guideline and
references shown.
Oxidation/Reduction Reactionsof Alcohols: Aldehydes, Ketones, Carboxylic Acids;
Alcohol, Epoxide, and Ether Reactions
Problems: 15.17, 15.19, 15.23, 15.27, 15.28 (d, e, f,h), 15.29 (a, b, c,d, e, fj, h),
15.32, 15.37, 15.38
Try 15.28 (g, i,j, k) and 15.29 (g, h, 1) with Extra Problem Set 6.
Properties, Nomenclature and Additional Reactions of Ethers and Epoxides
Problems: 16.21, 16.26, 16.28 - 16.31, 16.33, 16.34, 16.35
90
it
SC © CaH,Codca in the presence of pyridine
9
* w in the presence of pyridine
°
1528 back of he flowing scons as bien repo inthe chemical eae, Prt eh
Pralitin cach exe, showy seeechemisty alee apropcas.
OH x,
&@ Cl ot As A
ceceeng, {NNO 00409,
(COCCHI eco
bie
Cells
to Ei ihe Me
“Hono
© ‘o sat eet, oC
Coy
© CHEHCRCCH.C pe ca mC CHC,
ee j
g ow
© crdecnenmencian sesguinn, Ty
o
on ON, 9 on
© outa + eu niet £70,
7 wT
ow $
p
® + wodew,
° “um, = oer
0) Product ofan @) Sees o p
+ &
ae
(as Pri he pac! oman pods of enh of he lowing reasons. Specify Hees
operon One
cnc, Os Crt ig
6) CCH + NEON oy
if Ce Sen.ciy,
(6 eauetienen
CH CH, CH GMa
Nf
@ sec +
oCpestae Of"
\y,
BF Gin be be
© ae Oe Re
ad U o G.
© Cpe en cpa cH,
‘CHL as
®
w Sac (wo
afore
RO OTron
() CHs(CH) sCHAITs + CH,CH,CH,CB:SNa —>
1/1628 When (8).+)-2-phenyl2-bnaro! is allowed fo stand in metho containing a few drops
of aulfre acid racemic 2-mediexy-2-phenylbotane is formed. Suggest a reasonable mechanism
far this reaction
{716.29 Selestrenetion conditions that would allow you 1o cary out each of the following stereo-
speciic transformations:
H
@ yy + p12 propane
"
Act
oo) > (81,2 propanediol
(1630 The last step in the symthesis of divinyl ether (used as an anesthetic under the name
Vinethene) involves heating CICH,CH,OCH,CH.C} with potassitma hydroxide. Show how you
could srepare the necessary starting material CICH;CH,OCH,CH,Cl from ethylene
@
71547 Write chemical equations, showing all necessary reagents, for the preparation of t-butanol
ty tah afte owing eto tate ~on
(@ Hydroboraion-oxidation ofan alkene QA Fi20, fou" ttn
(2) Use of « Grgnad sagen Heo is
(© Ue of eign seagate aay ditsent om put gy ©) Ig re EL AAPA.
. het,
(Redotin cf weabonic aid <7
(0) Redan ofa mei exer 2 Ngee ST BARTER sayy
4) Reduction of a byt ever = L) aARou hile 2) woo eee? pe
(© Hydiogention of an sdehyde
6) Retain ih et yi Row “teas CAs 4) ak, He
hy Aa Mabie meee
¢H30H
15.19 Witte chemical equations, showing all necessary reagents, fo the preparation of tet-buty] Be fe Cte
sabol by: ZHAO
; 2) CHa ge +
(Reaction ofa Grigns reaget witha ketone >
Lethe
(© Reaction ofa Grigaré reagent with an ese of de ype RLOCH, 6) cg -octs + Ag Mghe 2 FPALO
\As23 Wie equuins showing How I phenylethanol (CQI:CHCH) could be prepared fm each ingle WOK, fetfrn
Gm: err _aw Nig
cf te foowing sang mauris a toy fe SF z. Hae
(6) Bromobenzene (@) Acctophenone L coe gg
(©) Benzaidchyde @ Bane — bY oye Tae Save
: (c) Benzyl alcohol, cH. xn)
9 oe Qs Dehr0, oh
45.27 Show how each of the following compounds can be synthesized from ¢yclopen
sy necessary ergs or Snags teats In ay cases fe Sate congound a be rade d) ON el Mote Leva)
owes ae Tee Nee bes
Br
Oa S
\ sexe
(9) 1-Preylecopensno
(b) 1-Phenylcyclopentene l nee
1
Or,
@ see lage Ey»
- C se “ © cavhenonnde a
(©) srans-2-Phenyleyelopentano
LG srpeny 5 petmetit
5.28 Wile the sructare ofthe penepal organic product formed in tbe reaction of I-propanal 4
wih each ofthe following reagents: me
(© Pyridiniom chiorochromate (PCC) in dichloromethane
© Resi eine 009 nae aeinme 2) YH ay oe
Sodium amide (SNH)
4) Sue 4) Q
xo ee ee agen chloride CHC OA,
w am \ soe ‘in the presence of pyridine JX CH, = Nols
+ ~e o> 3
o
o cno-€ \-ba inthe presence of pyridine dy au. orn, AY
ES
|As28 Pe pnp orp pot of cath of te felon mao Sty seme
‘chemistry where appropriate, me
CHACH, ¢ ROHS
o Dee cuca —
A T
3.
“—f OD
CH:
crc, Gh tty SF
Sb Siva > St—oensctts (no chenge af, chivas castes)
(0) CCH +
© CCHCCH BE CHa CHa QHZ CH
Oo”
oo) Cy t Chasis
J _. Se
oe plo NG
os OH
~Oeh= Chi,
Bt
® Ge Berm
ne (OH,
eo cnn wort
OE aan OE
‘CHACeHs oH
5 CH—CH,- OH
CHa CoH:
eS on Matas bi
w Ros SIE
) CAYCE) CLOTS + CHCHLCHCHNA—> CHSC CHa} ig CHa SCHACHaCHaCHs
BU
Dw D ene
116.28 When (R){+)-2-phenyi-2-butanol is allowed to stand in || containing a few drops ®
“een te i anny Totes ome. Sgpe + Rel an
hema one o (R)_OH team plucte
eine sain ttn awe ananttcttmaccrme —@ gs Jono
A Vig a Haosbx® ck Gents
(By) AES —> crys 2-propanedtiot fo chown be chine &
Yo one no eae
B by H26/ He
oK © (9).1,2-propanediol Thvergion ak Chirat © Roe S
e
\/r630 Tho tas step in the symhess of divinyl cher (used as an anesthetic under the mame
Vnethene) elves hesting CICH,CH,OCH,CH,CI with poasum hyaroxide. Show how Yow
could prepare the necessary starting material CICH,CH,OCE:CH,CS fom ee owseeet
' 50% O- CHG
Chis, “ee C= tig Ch OH —HeS28, C1 cHlaCHe
2
" [acid eodensetion. 9, alcohols)
®)
a.
: 030 ROD
2 QO ymio) Fetio
i 9
9 yy
e
OH
Hei ey) ON {
ore Ore Tae ry,
Othe
29 °
s : :
$0 cagboleyt, ine prsene of prize 2) Cute bo Uo non ke
ub .
oi EO 5
* 08 [7 in te presence of pyetine +) Hosa
‘ °°
V/4529 Bach of the fllowing reactions has been reported in the chemical literate. Prediet the
product in etch case, showing steeechomisy where appropsin.
@ cme 259,(€ 1) cit { pHs
Gis) = 4CH3)2
bu OH
0 CHcmc(cty, ACOH. OOseD,
(CH gsCOH, FIO”
° O- ne ction Cre exn at double bond)
© emquomciniv, gaahOOaae CHa PW SCC) HS
I
on
A i “ 9H tno Rawat
© catercrmcucrten, “ME, OL OOH CHEECH HZ CHCHs — oloulle bend)
ON : .
‘I cide —
@ cH + ( Nyy zai omy a 3y hos
Ce The in. stereochenistiey
: 90 / 9 °
rm it LS
* {COCCH _ = no ten,)
oe omc, C
" W
PN og a0) 5
é é dz (+ H20)
oe Con fee at &—OCHs
on’ oun?
9 9
si
gaa, goon CH CH,OH
ASC, ly
® hua,
a
cg A SxSHs04 sy g
ad aS + oy
“Gowee
AS.32, Suggest reaction sequences and reagents suitable for carrying out exch ofthe faowing con-
versions. Two syuthesi operations are required in each ease,
y MabHe, gon THE, (en)
“CHSOK 30H
Oo ates Cou He )
or HO.
Cena . Cr as why + y
®) CHAO [20
trod” ed
on g oH
‘ H Heso. CuHs (Re ca
oho » Ch oi rit gatas (fete
on len 2 2
“ott
#837 Colo living ee gatos y wing ttt fra ferconpemnes A [A= Q,, {sk QQ,
‘through
@ O® one caer = cao fee
coupon
Tisacno” CHO
ie Q
Compas + {oe Oe
° — Ga, Es cpr ES caco MMS cot, .c10
Compound
DMP Compound E compesnar L2)= Ray lew A)
On
» Oa Compound G Y&.&E°%> conpomnttt >
ess Viena ie ome 9 oe ens tig ter cette UD
se flowing ce fe
S 6d.
“CH Br
ot om
OF SO oso tle
caugenen, HS cate, es « 9 CHy-OH {zs
on
OCHACH, Sh Bee Ou OF CO
‘Sugeest # reasonable mechanisin for this reaction based on the observation that the ether produced Met (3,
Sh peal eve dahl rn ant eres can be on m9 be eee
cake) [F] = wine
(Compound
er i
Re Hasoy Gis Gus cnacon) \ FS
Cos pote Cote ny iChb > CellaGcHacHs TF" (Cts cect
ox, wis0 CH
aan tn 2282 a po ma geet
‘ethers are quite Hammable, their place in medical practice has been taken by highly halogenated
nonflasroabl eters. Two suc general anesthe agents te ufone and enfurane. These com
Pounds are fomeic: isfurane is shlovo-222-mifuoroeiy]duoromet ether eairane fe
2-chor-i ifoercehyditueromediyt sts, White the smuctal formulas of fsoforae aad
fens. a F Hq
isoflurane = Fo entlarane = Yoh ode
~ F-o5o5 0-8
eos (goer i ro) i
eH z F e
®
“is Soe sh fin cen eat uh fr rp ach fe owing
rele ne eee
. Ne
. _ Boon tt Luana on + cto Ae Bue
©) a wom | » “RAGS
ca PBrs or ae LO yt
oC tom tom ae
Jpenzene and cyclohexanol
OH Cros? oO
S imo?
Calls ) cr es Tie thee
(©) CQHCHACHCH, from bromobenzene and isopropyl aleabol i Wah. [2 He
bn See tat BOGE er > ve
(@) CgHSCH{CH,CH,OCH,CH; from beazyl alcohol and ethanol 2 2S; oH
See, (eet Pose Root SS Keats
ce) from 1,3-cyclohexadiene and ethsnol Celis
See (aot one Cc xe,
& . Cots
/ 16.3 Deduee the identity of the missing compounds in the following reaction sequences. Show
sereochemisny in parts (b) through (@).
A= Ch=CHcH-GHe OH
mcucirsie EM» Compound A Bs, Compound B
YO) Hemcucesbs ritse Chino, © (akan) a ,
sto" |rwwe BS
.<€51 Compound ©
7 “bee (CaHr0)
(Compound D
Vio, compeina (ep) 220, compamssiesiyos TL * —
Jowscowoe (alianis)
CH. CH “3g, Compound L (C7803)
(mp 995-101°C}
(sa et pen cf exp ib barely a
Cee ee ee racks more
OHs
Dave the structure of cingoe.
(635 The poluenesutfonate shown undergoes an intremolecular Williamson reaction on test-
‘ment with base to give 2 spirocycli ethes- Demonstrate your understanding of the terminology
seine neg sence hy wing eres ing eocheni oe pret 3
Ley OD, = 2
CHLCHEHLOTs > Cyto 7
Cos Cute
You might also like
- Mathnasium Application - Mubashir Razvi PDFDocument2 pagesMathnasium Application - Mubashir Razvi PDFnspegar92No ratings yet
- Carboxylic Acids and Carbonyls PDFDocument10 pagesCarboxylic Acids and Carbonyls PDFnspegar92No ratings yet
- Chem 247 Enols and Nomenclature PDFDocument9 pagesChem 247 Enols and Nomenclature PDFnspegar92No ratings yet
- Organic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFDocument9 pagesOrganic 1 Alkyl Halides, Free Radicals, SN1 SN2 PDFnspegar92No ratings yet
- Organometallics, Epoxides, and Ethers Practice PDFDocument6 pagesOrganometallics, Epoxides, and Ethers Practice PDFnspegar92No ratings yet
- Electrophic Aromatic Substitution Practice PDFDocument5 pagesElectrophic Aromatic Substitution Practice PDFnspegar92No ratings yet
- Electrophilic Addition Practice 1 PDFDocument4 pagesElectrophilic Addition Practice 1 PDFnspegar92No ratings yet
- General Reactions Practice PDFDocument5 pagesGeneral Reactions Practice PDFnspegar92No ratings yet
- Dienes and Diels-Alder Practice PDFDocument4 pagesDienes and Diels-Alder Practice PDFnspegar92No ratings yet
- Chem 247 Aromatics and Electrophilic Aromatic Substitution PDFDocument9 pagesChem 247 Aromatics and Electrophilic Aromatic Substitution PDFnspegar92No ratings yet
- Chem 247 Organometallic and Grignard Reactions PDFDocument5 pagesChem 247 Organometallic and Grignard Reactions PDFnspegar92No ratings yet
- Aromatic Nomenclature and Concepts PDFDocument3 pagesAromatic Nomenclature and Concepts PDFnspegar92No ratings yet
- Chem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFDocument3 pagesChem 247 Aldehydes, Ketones, and Carbonyl Reactions PDFnspegar92No ratings yet
- Aldehydes and Ketones Practice PDFDocument9 pagesAldehydes and Ketones Practice PDFnspegar92No ratings yet
- Alkyl Halides Practice PDFDocument9 pagesAlkyl Halides Practice PDFnspegar92No ratings yet
- Chem 247 Alkynes, Conjugation, and Diels-Alder PDFDocument7 pagesChem 247 Alkynes, Conjugation, and Diels-Alder PDFnspegar92No ratings yet
- Chem 247 Exam 3 PDFDocument3 pagesChem 247 Exam 3 PDFnspegar92No ratings yet
- Alcohols, Ethers, Thiols, and SulfidesDocument6 pagesAlcohols, Ethers, Thiols, and Sulfidesnspegar92No ratings yet
- Alkynes Practice PDFDocument3 pagesAlkynes Practice PDFnspegar92No ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)